On
the neural computation of utility: implications from studies of
brain stimulation reward
On the neural computation of utility:
implications from studies of brain stimulation reward
Peter Shizgal
Center for Studies in Behavioral
Neurobiology
Concordia University
| To appear in the forthcoming Russell Sage Foundation
title: D. Kahneman, E. Diener & N. Schwarz, Eds. Foundations
of hedonic psychology: Scientific perspectives on
enjoyment and suffering. (Reproduced with permission
of the Russell Sage Foundation.) |
Acknowledgments
The author is grateful to Andreas Arvanitogiannis, Kent
Berridge, Kent Conover, Randy Gallistel, Bart Hoebel, Daniel
Kahneman, and Roy Wise for their helpful comments and to the
"Fonds pour la Formation de Chercheurs et l'Aide à la
Recherche du Québec" (grants ER-0124 and CE-0103), the
Medical Research Council of Canada (grant MT-8037), and the
Natural Sciences and Engineering Research Council of Canada
(grant A0308) for research support.
Summary
1. Like other vertebrates, from goldfish to humans, rats
will work in order to deliver electrical stimulation to
certain brain sites. Although the stimulation produces no
evident physiological benefit, it is sought out avidly, as if
it were a biologically significant resource. Thus, it has
long been thought that the rewarding stimulation activates
neural circuitry involved in the evaluation and selection of
goals.
2. Computing the utility of goal objects involves a
tightly integrated set of perceptual, cognitive, and
motivational mechanisms. I argue that rewarding electrical
brain stimulation engages only a subset of these mechanisms.
If so, comparison of the ways in which the utility of
electrical brain stimulation and natural reinforcers are
computed may highlight operating principles and isolate
components of the computational mechanisms.
3. In the view proposed here, information about goal
objects and consummatory acts is processed, in parallel, in
three different channels.
3.1. Perceptual processing indicates what and where
the goal object is
3.2. A stopwatch-like interval timer predicts when or
how often the goal object will be available.
3.3. Under the influence of information about the
current physiological state, an evaluative channel
returns a subjective weighting of strength variables such
as the concentration of a sucrose solution or the
temperature of an air current.
3.4. The output of these channels is recorded in
multidimensional records that include
3.4.1. information of perceptual origin about
amount and kind (e.g., food, water, or salt)
3.4.2. information from the timer about rate and
delay
3.4.3. a subjective assessment of intensity
provided by the evaluative channel
4. This chapter addresses the relationships between brain
stimulation reward (BSR), the perceptual, interval timing,
and evaluative channels, and the variants of utility proposed
by Kahneman and his coworkers on the basis of their studies
of evaluation and choice in human subjects.
4.1. It is argued that the output of the evaluative
channel can be manifested in experience as pleasure or
suffering but that awareness is not necessary in order
for this signal to influence action.
5. The neural signal injected by rewarding electrical
stimulation is portrayed as providing meaningful information
about rate, delay and intensity but not about amount or kind.
This proposal is used to account for
5.1. competition and summation between BSR and natural
rewards
5.2. differential effects of physiological feedback on
the utility of BSR and natural rewards
5.3. matching of behavioral allocation to the relative
rates and intensities of BSR
5.4. differences in the elasticity of demand for BSR
and food in a closed economy
5.5. the high substitutability of BSR for food and
water in an open economy
6. The powerful aftereffect of BSR that potentiates
efforts to obtain additional stimulation is related to
expectancy.
Brain stimulation
reward
A rat sits quietly in the start box of a runway, its access to
the 6-ft alley blocked by a acrylic panel. The rat begins to
groom, licking its paws and rubbing them over its snout. As the
fastidious creature cleans the top of its head, it encounters a
miniature electrical connector fastened firmly to its skull; the
rat’s paws sweep across this now-familiar appendage without
breaking rhythm. Unhindered by the flexible cable linking the
connector to a stimulator, the rat then turns its head to groom
its flank.
The grooming bout is cut short as the stimulator sends small
surges of current through an electrode attached to the connector.
Deep in the brain, these stimulation pulses trigger salvos of
impulses in nerve fibers coursing past the electrode tip. The rat
looks up, highly alert. It stretches forward and explores the
start box, sniffing the floor and scanning its head from side to
side. At the offset of the stimulation, the rat approaches the
acrylic panel, seizes the top with its forepaws, and hops as if
trying to vault the barrier. Shortly thereafter, a solenoid
withdraws the panel, and the rat explodes into the alley like a
sprinter quick out of the blocks. It races to the goal box, and
without breaking stride, presses a lever that has triggered
delivery of stimulation pulses on previous trials.
On this trial, the rat is disappointed: The experimenter has
turned off the stimulation in the goal box. Accustomed to such
betrayals, the rat adjusts to the new conditions within a few
trials. Although the stimulation in the start box is unchanged,
it now fails to galvanize the rat’s behavior. No longer
expecting to receive stimulation in the goal box, the rat remains
in the start box following removal of the barrier, and after
casually sniffing the slot into which the barrier has been
withdrawn, the rat lies down and yawns.
In this vignette, the rat treats the stimulation much as if it
were a biologically significant resource, such as food during a
period of limited availability or a heated nest box in a cold
environment. Given that such natural resources serve as highly
effective inducements for learning and performance, the effect of
the stimulation that the rat seeks to reinstate is called
"brain stimulation reward" (BSR).
The phenomenon of BSR has been observed in a wide variety of
vertebrates, from goldfish to humans (Bishop, Elder, & Heath,
1963; Boyd & Gardiner, 1962; Distel, 1978; Lilly &
Miller, 1962; Olds & Milner, 1954; Porter, Conrad, &
Brady, 1959; Roberts, 1958). Unlike the case of natural
reinforcers, the rewarding effect of electrical brain stimulation
is not undermined by recent "consumption," and no prior
deprivation of essential physiological resources is required in
order to elicit and maintain vigorous performance. Among the
feats that rats will perform to obtain the rewarding stimulation
are running uphill while leaping over hurdles (Edmonds &
Gallistel, 1974) and crossing an electrified grid (Olds, 1958).
When brief, intense trains of lateral hypothalamic (LH)
stimulation are available continuously, rats will work for the
rewarding stimulation at the expense of forgoing their sole daily
opportunity to eat (Frank & Stutz, 1984; Routtenberg &
Lindy, 1965) and will prefer such stimulation to water, even
following prolonged fluid deprivation (Morgan & Mogenson,
1966). Many abused drugs, such as cocaine, amphetamine, heroin,
cannabis, and nicotine, potentiate the rewarding effect of the
stimulation (Wise, 1996).
The rewarding effect of electrical brain stimulation has long
been linked to the subject of this volume: the scientific study
of enjoyment and suffering. Indeed, the first report of BSR in
the press (Macfarlane, 1954) heralded the discovery of a
"pleasure area" in the brain, and similar phrases were
used in early scientific publications (Olds, 1956). However,
subsequent developments, both in the study of BSR and in the
study of the relationship between hedonic experience and choice,
argue for a richer and more nuanced characterization.
Toward a
new view of brain stimulation reward
In this chapter, I summarize and extend a new view of the
neural signal mimicked by the rewarding stimulation (Shizgal,
1997; Shizgal & Conover, 1996). This view is rooted in a
long-standing idea: that multiple modes of processing are brought
into play in parallel when an animal encounters a goal object
(e.g.(Pfaffmann, Norgren, & Grill, 1977; Zajonc, 1980)).
Perceptual processing determines the identity, location, and
physical properties of the goal object, whereas the information
derived from evaluative processing is used to determine what the
goal object is worth. A third processor, which acts as a
stopwatch timer, is concerned with when or how often the goal
object will be available (Gibbon, 1977; Gibbon, Church,
Fairhurst, & Kacelnik, 1988). Kent Conover and I have
proposed that BSR arises from activation of the evaluative system
(Shizgal, 1997; Shizgal & Conover, 1996). In our view, the
stimulation simulates a meaningful signal in this system and also
provides an interpretable input to the timer. The evaluative and
timing systems provide sufficient information for computing a
payoff. Thus, the rat is drawn back to the site where stimulation
was delivered previously and incited to repeat the acts that
trigger the stimulation. In contrast, the stimulation-induced
signals are not interpretable by the perceptual system. If so,
the rewarding stimulation cannot recreate the perceptual
experience produced by contact with a natural goal object. In
effect, the rat knows that the lever delivers something valuable,
but it cannot determine what that something is.
The account developed here embeds ideas that Conover and I
derived from studying BSR and gustatory reward in rats within a
broader conception of how utility is computed and links these
ideas to concepts drawn from the study of hedonic experience in
humans. From this perspective, there are several ways in which
the analysis of BSR might advance the scientific study of
enjoyment: 1) The natural stimuli that give rise to enjoyment act
on the perceptual, evaluative, and timing systems. If rewarding
brain stimulation indeed mimics only a subset of these effects,
then it can serve as a tool to tease apart what is normally a
tightly-integrated complex of responses, helping isolate
individual components so that their properties can be described
and understood. 2) In the view presented here, the neural signals
mimicked by BSR bias the subject to resist interruption of
pleasurable activities and to repeat actions that have led to
pleasant consequences in the past. If so, a thorough
understanding of the processes underlying BSR should help explain
how the evaluative system steers ongoing behavior and influences
both the amount and kinds of enjoyment that will be experienced
in the future. 3) The neural signals underlying BSR arise from an
observable volley of nerve impulses elicited at a known location
in the central nervous system. Thus, the phenomenon of BSR should
be particularly propitious for attempts to understand evaluative
processing in terms of the activity of identified neural
circuitry. (For reviews of research aimed at identifying the
neural circuitry subserving BSR, see (Shizgal, 1997; Shizgal
& Murray, 1989; Yeomans, 1988).)
Variants
of utility
In recent years, Kahneman and his coworkers have investigated
the relationship between choice and the operation of the
evaluative system, delineating several variants of utility
(Kahneman, 1994; Kahneman, Wakker, & Sarin, 1997). Their work
has been carried out with human subjects, whereas research on BSR
has been conducted almost exclusively on rats and other
laboratory animals. Nonetheless, the distinctions drawn by
Kahneman et al. between different types of utility and their
proposals as to how variants of utility are interrelated provide
a very useful framework for linking research on BSR to more
general conceptions of choice and to hedonic experience.
Instantaneous utility.
We continuously evaluate the stream of sensory experience and
adjust our behavior accordingly. Kahneman et al. (1997) refer to
the product of such ongoing evaluation as "instant
utility," a quantity that can vary in sign and magnitude.
They give the term two connotations, one defined with respect to
hedonic response and the other with respect to action. According
to this view, instant utility is experienced along an opponent
hedonic dimension ("good/bad") while biasing the
individual to continue or terminate the current course of action.
States and stimuli that produce positive values of instant
utility are experienced as pleasurable while impelling us to
continue what we are doing; states and stimuli that produce
negative values have the opposite effects. Like the brightness of
a visual stimulus at a particular point in time (Schreiber &
Kahneman, submitted), instant utility is a property of the
moment. I shall refer to this quantity as "instantaneous
utility."
Remembered utility.
During experiences such a meal at a fine restaurant or a visit to
the theater, instantaneous utility fluctuates over time. Despite
the complexity of the resulting temporal profiles, we have little
difficulty in applying single ratings to such experiences such as
"four stars out of a possible five" or in reporting to
others whether we obtained our money’s worth. Kahneman et
al., refer to such unitary ratings of temporally extended
experiences as "remembered utility." They propose that
in compressing a temporal profile of instantaneous utility into a
remembered utility, we apply heuristics that simplify the task,
speed its execution, and minimize the mnemonic and computational
resources required.
Decision utility.
Remembered utility influences behavior via a further computation,
the calculation of the weights applied to the different outcomes
of a decision under active consideration. Kahneman et al. call
these weights "decision utilities" (Kahneman, 1994;
Kahneman et al., 1997). In their terms, the rat’s choice of
whether or not to leave the start box would be said to depend on
the decision utilities of two outcomes, obtaining additional
stimulation in the goal box or lying down and resting in place.
Predicted utility.
Kahneman et al. point out that the calculation of decision
utility may reflect not only remembered utilities but also
additional factors, such as the "predicted utility" of
the outcome. Whereas remembered utility returns the overall
"goodness" of the last meal we ate at a particular
restaurant, predicted utility reflects our expectation of how
much we will enjoy a visit to that restaurant today.
Relationship
of BSR to different variants of utility
How is the ongoing neural activity driven by rewarding
electrical stimulation related to instantaneous utility?
According to the proposal advanced by Conover and Shizgal, the
rewarding stimulation achieves its grip over ongoing behavior by
simulating the real-time effect of a natural reward on the
evaluative system, i.e., by driving instantaneous utility to
positive values. I propose that this signal can steer behavior in
the absence of awareness but does not do so invariably. Via the
allocation of attentional resources, the instantaneous utility
signal can gain access to working memory and may be manifested in
human experience as pleasure or suffering. Thus, the dual meaning
imparted to instantaneous utility by Kahneman and his coworkers
has been retained, but the link between the action component and
the hedonic component is weakened, with the action component
treated as the more fundamental. Some advantages of allowing the
instantaneous utility signal to impinge on awareness are
discussed below.
In the vignette at the beginning of this chapter, the behavior
of the rat depends on whether or not it has received sufficiently
rewarding stimulation in the goal box on preceding trials. Thus,
the rat appears to have recorded the utility of the stimulation
received previously. We will see shortly that there is a striking
similarity in the way that the instantaneous utilities of BSR in
rats and certain temporally extended experiences in humans are
translated into remembered utilities.
Records of payoff are inherently multidimensional and may well
be derived from multiple modes of processing. I argue below that
what Kahneman et al. call remembered utility captures the
subjective "intensity" of the reinforcer, which is but
one of several components of such records. The evaluative channel
that assesses intensity is complemented by a stopwatch timer that
delivers assessments of encounter rate and delay and by
perceptual mechanisms that can return estimates of amount (e.g.,
the mass of an acorn) and kind (e.g. food versus water).
Information about kind can be used to determine the degree to
which one reinforcer can substitute for another. A key tenet of
behavioral economics is that substitutability determines whether
and how much behavioral allocation will shift from one reinforcer
to another in the face of price changes. Moreover, it can be
argued that the elasticity of demand for a particular kind of
resource depends on additional information of perceptual origin:
environmental distribution of that resource in the environment.
In this view, decision utilities are derived from a
combination of perceptual, timing, and evaluative data. If BSR
indeed reflects meaningful signals in the evaluative and timing
systems in the absence of meaningful perceptual information, then
performance for BSR should respond differently to economic
constraints than performance for natural reinforcers. The
literature reviewed below is interpreted to support this
contention and to suggest that comparisons between performance
for BSR and for natural reinforcers shed light on the
psychological resources involved in computing decision utilities.
In the opening vignette, the start-box stimulation produces an
aftereffect that potentiates behavior aimed at procuring
additional reward. If the rat has been given a free
"taste" of the stimulation at the start of the trial,
it will show more pronounced anticipatory behaviors prior to the
removal of the barrier and will run down the alley faster once
allowed to enter. This is reminiscent of the way that savoring a
particularly tasty hors-d’oeuvre at a reception can incite
visual search for the waiter and vigorous pursuit once he
reappears. Just as the anticipatory search and the pursuit of the
waiter depend on the expectation that the supply of
hors-d’oeuvres has not yet been exhausted, the anticipatory
behavior of the rat in the start box depends on the expectation
that stimulation will be available in the goal box. Such
expectations may be related to the predicted utilities discussed
by Kahneman et al. in that anticipation of a future event
influences present choices.
In order to be manifested in behavior, a decision utility must
be processed by a selection rule. "Choose the largest,"
will be assumed as the rule. The question of how the resulting
decisions are translated into action is beyond the scope of this
chapter; the reader is referred to Gallistel’s Organization
of Action (1980) for a fine introduction to this topic.
In the following sections, the relationship between BSR and
the variants of utility proposed by Kahneman et al. is discussed
in detail. Exploration of this relationship casts BSR data in a
new light and suggests new directions for future research.
BSR basics
Some basic characteristics of the electrical stimulus and the
neural circuitry responsible for its rewarding effect must be
described before developing the arguments linking BSR to
different variants of utility. The stimuli used in most modern
BSR experiments consist of trains of short-duration current
pulses. With pulse duration held constant, the strength of a
train is determined by pulse amplitude (current) and frequency.
The greater the current, the larger the number of neurons
directly stimulated by the electrode. Over the ranges of
frequencies used in most studies, each directly stimulated neuron
can be assumed to fire once per pulse. Thus, as depicted in
Figure 1, each pulse produces a synchronous volley of nerve
impulses (action potentials) in the population of
directly-stimulated cells that give rise to the rewarding effect,
and the aggregate firing rate of this population is determined by
the stimulation current and frequency. It is highly unlikely that
this population of neurons responds in such a synchronous manner
to any natural stimulus, yet the artificial stimulation does
mimic some of the properties of a natural reinforcer. As
discussed below, this provides a clue as to how information is
represented in the neural system underlying the rewarding effect.
The counter model. The
firings of the directly stimulated neurons appear to be
translated into the rewarding effect in a surprisingly simple
manner. With the duration of a stimulation train held constant,
the strength of the rewarding effect appears to depend only on
the aggregate rate of firing in this population of directly
stimulated cells. According to this "counter model"
(Gallistel, 1978; Gallistel, Shizgal, & Yeomans, 1981;
Simmons & Gallistel, 1994), it matters not whether one
hundred directly stimulated neurons fire ten times each during a
particular time window or whether twenty neurons fire fifty times
each. The rewarding impact of the stimulation will be the same
provided that aggregate impulse flow is constant. If activity
elicited in these neurons by natural stimuli is integrated in the
same manner, then the synchronous firings triggered by the
artificial stimulation should produce the same effect as an
equivalent number of asynchronous firings triggered by a natural
stimulus.
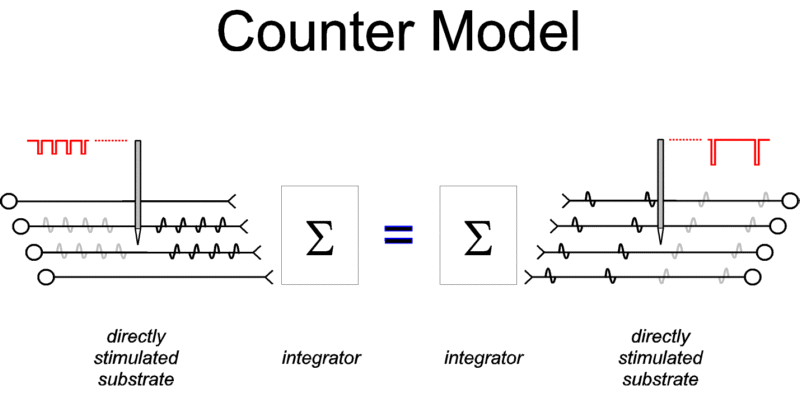 |
Figure 1: The counter model. Action potentials
elicited in the directly activated neurons responsible
for BSR impinge on a neural circuit that integrates their
effects over time and space. The output of this
integrator is determined by the aggregate rate of firing
during a fixed time window. Thus, firing two neurons four
times each produces the same output as firing four
neurons twice each. (In addition to triggering action
potentials that propagate to the synaptic terminals, the
stimulation also triggers action potentials that
propagate "backwards," toward the cell body.
These "antidromic" action potentials, shown in
gray, will have no behavioral effect unless they invade
another axonal branch.) |
The counter model is shown in Figure 1. The directly
stimulated neurons responsible for the rewarding effect are
depicted as providing input to an "integrator" that
combines the effects of incoming action potentials over time and
space. The output of the integrator is determined by the
aggregate rate of firing at its input. It is argued here that
this output is the sole determinant of the instantaneous utility
of the stimulation and that the remembered utility of the
stimulation is derived from certain "exemplar values"
(Schreiber & Kahneman, submitted) of its instantaneous
utility.
Instantaneous
utility, resistance to interruption, and BSR
At many brain sites, BSR is accompanied by aversive side
effects, which are due to the activation of different neurons
than the ones responsible for the rewarding effect (Bielajew
& Shizgal, 1980; Bower & Miller, 1958; Shizgal &
Matthews, 1977). By judicious selection of stimulation site,
current, frequency, and temporal pattern, the aversive
side-effects can be minimized. When such precautions are taken,
the rat will readily press a lever to initiate a long-duration
train of stimulation but will not press a second lever that turns
off the stimulation (Shizgal & Matthews, 1977). If the
experimenter interrupts such a train, the rat will immediately
rush over to the lever and reinitiate the stimulation. This
suggests that if given the opportunity, the rat would strive to
prevent interruption of such stimulation. If so, it should prove
possible to measure the action component of instantaneous utility
in real time by assessing the commitment of the rat to keeping
the current flowing.
To my knowledge, such an experiment has not been done. A
promising way to perform it would be to use the temptation of an
alternative reward to assess the rat’s commitment to the
ongoing stimulation. At different times during the delivery of a
long train, a choice would be offered between two options: a)
continuation of the train or b) immediate cessation of the train
coupled with delivery of an alternate reward. The strength of the
alternate reward required to tempt the rat to terminate the long
train would provide a measure of the instantaneous utility of the
ongoing stimulation at the moment of choice. In principle, such a
measure would provide a direct test of the prediction that both
the remembered utility of the stimulation and its instantaneous
utility are derived from the output of one and the same
integrator.
Instantaneous utility, hedonic experience, and
BSR
We experience pleasure and pain as powerful, adaptive
influences on our behavior. As Bentham put it so memorably
(Bentham, 1996 (originally published: 1789)):
"Nature has placed mankind under the governance
of two sovereign masters, pain and pleasure. It is for
them alone to point out what we ought to do, as well as
to determine what we shall do."
Bentham’s assertion that pleasure and pain direct
action may ring so true to our experience as to lead us to the
converse view: that action reflects hedonic state. If
pleasure and pain lead us to seek out or maintain contact with a
pleasurable stimulus and to avoid or interrupt contact with a
painful one, then is it not justified to infer these experiences
from observation of the acts they promote? It is in this sense
that the description of BSR sites as "pleasure areas"
has had an intuitive appeal to many. If the rat is willing to
work so hard to initiate the electrical stimulation, then must
not the stimulation be pleasurable?
In my view, the answer to both questions is no, not
necessarily. I will argue that the two components of
instantaneous utility can coincide, but that they need not do so
invariably. Thus, the link between hedonic experience and the
control of action is less direct in the account presented here
than in Bentham’s original formulation or in modern
developments of Bentham’s position (Cabanac, 1992). I
speculate below on what may be gained by supplementing the
action-oriented component of instantaneous utility with a hedonic
response.
To turn Bentham’s formulation around and to infer
pleasure and pain from behavior is to make a strong assumption:
that actions such as resistance to interruption of a stimulus or
attempts to escape from it cannot be produced in the absence of a
hedonic response, i.e., without the express consent of the
"sovereign." ("It is for them alone …
to determine what we shall do.") By labeling a state as
pleasurable or painful, we imply that we are aware of it;
"unconscious pleasure" and "unconscious
suffering" are oxymorons. If so, asserting that a hedonic
response is a necessary condition for resistance to interruption
or escape is tantamount to stating that such actions cannot be
produced in the absence of awareness.
Bentham’s position does not stand up well in the face of
a large body of psychological research and theory that treats
much of the foundation of perception, thought, emotion and action
as hidden from awareness (Baars, 1988; LeDoux, 1996; Nisbett
& Wilson, 1977). In such views, consciousness depends on the
serial operation of a limited-capacity process. Rather than
forcing all signals vying for the control of action to pass
through this processing bottleneck, much of the task of real-time
control is assigned to a collection of specialized lower-level
processors operating in parallel and in the absence of awareness
(Baars, 1988). If so, the hedonic and action-oriented components
of instantaneous utility are dissociable, and we cannot
necessarily infer the hedonic content of experience on the basis
of behavioral observation alone.
In human subjects, we can address the relationship between
hedonic experience and the control of action empirically, via
methods for concurrent measurement of self-ratings and behavior.
For example, ratings of the sign and intensity of hedonic
experience can be collected while observing whether the subject
maintains or breaks off contact with a stimulus. It is not
surprising that strong correlations have been noted in such
studies between subjective hedonic ratings and measures of choice
(Cabanac & LeBlanc, 1983). However, dissociations have been
noted as well. For example, in a study of heroin addicts
self-administering morphine, low doses of the drug were
vigorously self-administered despite subjective ratings of zero
on both a monetary value scale and a Likert scale of liking; a
saline solution received similar subjective ratings but was not
self-administered (Lamb et al., 1991). It is interesting to note
that increasing the dose of morphine brought the subjective
ratings into accord with the behavioral measure. Thus,
self-ratings of hedonic response could coincide with action but
did not do so invariably. (The reader is referred to the chapter
in this volume by Berridge for an alternative interpretation of
these data.)
In contrast to the tools available for studying the
relationship between hedonic experience and action in humans, we
do not have well-validated and general means for measuring
enjoyment and suffering in non-human animals. Although it should
prove possible, as proposed above, to measure the rat’s
resistance to interruption of the stimulation, we cannot be sure
how the rat feels while the current is flowing. Nonetheless, I
will propose that the relationship between the control of ongoing
behavior and processes that contribute to awareness in humans
could be investigated by neurobiological means in non-human
subjects.
In the view elaborated here, which borrows from proposals by
Ledoux (1996), the output of the neural process that determines
whether an action will be continued or terminated (the
"continue/stop signal:" the action-oriented component
of instantaneous utility) is not isomorphic with pleasure or
suffering but will be manifested in awareness as a hedonic
response if the continue/stop signal gains access to working
memory. This access is gated by attention and will be most likely
to occur when the action-oriented signal and associated stimuli
attain high values. Nonetheless, sufficient allocation of
attention might allow weaker signals to trigger a hedonic
response, and strong signals might fail to do so in the absence
of attention, e.g., when events are highly predictable and
behavioral responses are highly practiced. When the continue/stop
signal does succeed in breaching the waterline of awareness, it
can marshal further attentional resources and direct planning
while coordinating the activity of processes that operate beyond
the margins of conscious experience.
To develop the argument, let us assume that we were given the
task of designing a robot that simulates the behavior of a rat.
Ongoing action is controlled by a continue/stop signal derived
from real-time information about external stimuli and from the
state of the internal environment. For example, when body
temperature is low and a warm microenvironment is encountered,
the continue/stop signal will have a positive value, thus
promoting continued contact with the heat source and a return to
thermal homeostasis; when internal temperature is too high, the
same thermal stimulus will drive the continue/stop signal to
negative values and terminate contact. This adjustment of the
neutral point as a function of internal state reflects
Cabanac’s concept of alliesthesia (Cabanac, 1971).
The sensory and evaluative mechanisms that generate the
continue/stop signal should allow the robot to simulate certain
adaptive responses of a rat to ongoing sensory stimulation.
However, the robot would need additional circuitry in order to
mimic abilities that figure prominently in cognitively-oriented
accounts of goal-directed behavior such as the resolution of
conflicts between multiple goal-related stimuli by means of
selective allocation of attention, navigation in space using
stored representations, and planning a route leading from the
current state to a higher-valued one. In an influential treatment
of animal navigation (Gallistel, 1990), an egocentric spatial
representation of the current environment is constructed from
successively encountered stimuli and then translated into
geocentric coordinates using stored information about the
position of vantage points and angles of view. Essential to such
tasks is a readily accessible ("working") memory store,
in which critical information is held on-line. Working memory
also plays an essential role in models that find efficient routes
to goals (Gallistel, 1990; Johnson-Laird, 1988; Miller, Galanter,
& Pribram, 1960); access to this limited-capacity store is
gated by attention. If we were to incorporate attention, working
memory, and a route-finding mechanism in our robot, would it be
advantageous to allow the continue/stop signal to interact with
them, and if so, how?
For help in addressing this question, let us enlist the
assistance of a wise student of behavior, the novelist, Joseph
Heller. In Catch-22 (Heller, 1961), Heller explores the
relationship between instantaneous utility and the control of
action. Heller’s antihero, Yossarian, is a World War II
bombardier surrounded by suffering, death, and destruction.
Desperate to survive his military service, Yossarian refuses to
accept that things are as they ought to be. During a
philosophical argument about the failings of the Supreme Being,
Yossarian complains:
"Why in the world did He ever create pain? …
Why couldn’t He have used a doorbell instead to
notify us, or one of his celestial choirs? Or a system of
blue-and-red neon tubes right in the middle of each
person’s forehead. Any jukebox manufacturer worth
his salt could have done that. Why couldn’t
He?"
Yossarian wants to believe that the protective function of
pain could be fulfilled by a warning signal that is merely
informative. But what would confer upon such a signal the ability
to capture attention, wrest control of planning, and coordinate
the multiple processes controlling action? Sadly for Yossarian
and his flak-riddled comrades, the insistent unpleasantness of
pain is a highly effective means of achieving these ends. Gladly
for the rest of us, so too is the shock of intense pleasure.
With our response to Yossarian in mind, let us return to our
robotic rat and implement a key improvement. We will now arrange
things so the continue/stop signal can access working memory and
influence the allocation of attention. The continue/stop signal
will attain large negative values in response to ongoing or
imminent tissue damage; focusing attention on such a signal would
tend to promote it to the top of the planning agenda and reduce
the odds that competing stimuli would divert scarce cognitive
resources from the task of terminating the noxious input. In
contrast, the continue/stop signal will register large positive
values in response to contact with potentially beneficial stimuli
such as food sources or locations that promote maintenance of
thermal neutrality. The likelihood of interrupting contact with
the beneficial input would be reduced by allowing it to draw
attention away from competing stimuli. Temporarily suppressing
planning might also help "lock in" contact with an
input that is driving the continue/stop signal to large positive
values.
Working memory and attentional control of its input are
regarded as key components of the foundation for awareness in
humans (Baars, 1988; Johnson-Laird, 1988; LeDoux, 1996). Thus, if
this sketch were generalized from our robotic rat to ourselves,
we should predict that extremes of instantaneous utility (strong
continue/stop signals) would tend to be reflected in our
experience as well as in our behavior. Stimuli that produce
weaker excursions of instantaneous utility might, nonetheless,
exercise behavioral control, but, as in the case of the low doses
of morphine self-administered by the addicts, such stimuli are
less likely to be manifested in awareness.
It has been argued that consciousness enables the
"broadcasting" of information throughout the cognitive
architecture to the many specialized processors that operate
beyond the margins of awareness (Baars, 1988). If so, expressing
the continue/stop signal in awareness as pleasure or pain would
help marshal and coordinate the activity of multiple cognitive
processes in mounting a highly integrated response to the
eliciting stimuli.
I propose below that direct electrical stimulation of certain
brain regions can mimic the effect of a naturally occurring
stimulus on the neural circuitry that computes instantaneous
utility. If so, the argument developed above predicts that such
stimulation should be able to drive instantaneous utility to
levels that can impinge on awareness in humans. Indeed, when
direct electrical stimulation has been delivered to some of the
brain regions homologous to sites where BSR is obtained in rats,
its effect has been described by human subjects as pleasurable
(Heath, 1964).
By means of electrophysiological recordings, the activity of
neurons implicated in working memory is monitored routinely in
non-human animals (Goldman-Rakic, 1996; Watanabe, 1996), and the
modulating effects of attention can be observed (Treue &
Maunsell, 1996). Thus, it may prove possible to determine, by
conventional neurobiological means, whether instantaneous utility
signals can capture attentional resources and gain access to
working memory. Research on BSR could play a crucial role in such
experiments by identifying neural circuitry subserving
instantaneous utility and by providing a potent means of
controlling it.
Instantaneous utility is a property of the moment, and thus,
this discussion had been focused on real-time processing. Let us
now turn to a process that bridges the present and the future:
translation of instantaneous utility into a stored record. The
bulk of the research carried out on BSR has probed such records.
Transformation
of instantaneous utility into remembered utility
In Kahneman’s portrayal, remembered utility is derived
from a temporal profile of instantaneous utility. The
continuously fluctuating value of instantaneous utility during a
temporally extended experience is compressed into a single
remembered utility on which future decision weights can be based.
Imagine, for example, that upon pressing the lever in the goal
box, the rat receives a prolonged train of stimulation that waxes
and wanes in strength over several minutes (e.g. (Lepore &
Franklin, 1992)), much as the level of a drug in the bloodstream
rises and falls following administration. How does the rat
compress this temporally extended experience over time so as to
derive a single decision utility that can weight future choices?
One way to derive a single remembered utility from a
temporally extended experience is to compute the temporal
integral or the average of the entire sequence of instantaneous
utilities. Kahneman and his coworkers propose a very different
and much simpler strategy. Their human subjects appear to extract
two key values from the temporal profile: the peak instantaneous
utility and the instantaneous utility at the end of the
experience; some intermediate value, such as the average of the
peak and end values, is then used as the remembered utility
(Kahneman, Fredrickson, Schreiber, & Redelmeier, 1993;
Redelmeier & Kahneman, 1996). Only in unusual circumstances
would the peak-and-end rule be expected to generate a result
radically different from the outcome of temporal integration.
However, a simple rule of thumb, such as peak-and-end averaging,
should be executed more quickly than retrospective temporal
integration, while consuming fewer mnemonic and computational
resources.
If the peak-and-end heuristic were employed, then remembered
utility should be insensitive to variations in the duration of
the temporally extended experience. This prediction has been
confirmed in both experimental and observational studies carried
out with human subjects (Fredrickson & Kahneman, 1993;
Kahneman et al., 1993; Redelmeier & Kahneman, 1996). For
example, in retrospective evaluations of colonoscopy procedures
that varied in duration from 4 - 67 minutes,
aversiveness was not correlated with duration, but was strongly
correlated with real-time ratings of both peak pain and pain at
the end of the procedure (Redelmeier & Kahneman, 1996). Such
insensitivity to duration has been called "duration
neglect" (Fredrickson & Kahneman, 1993).
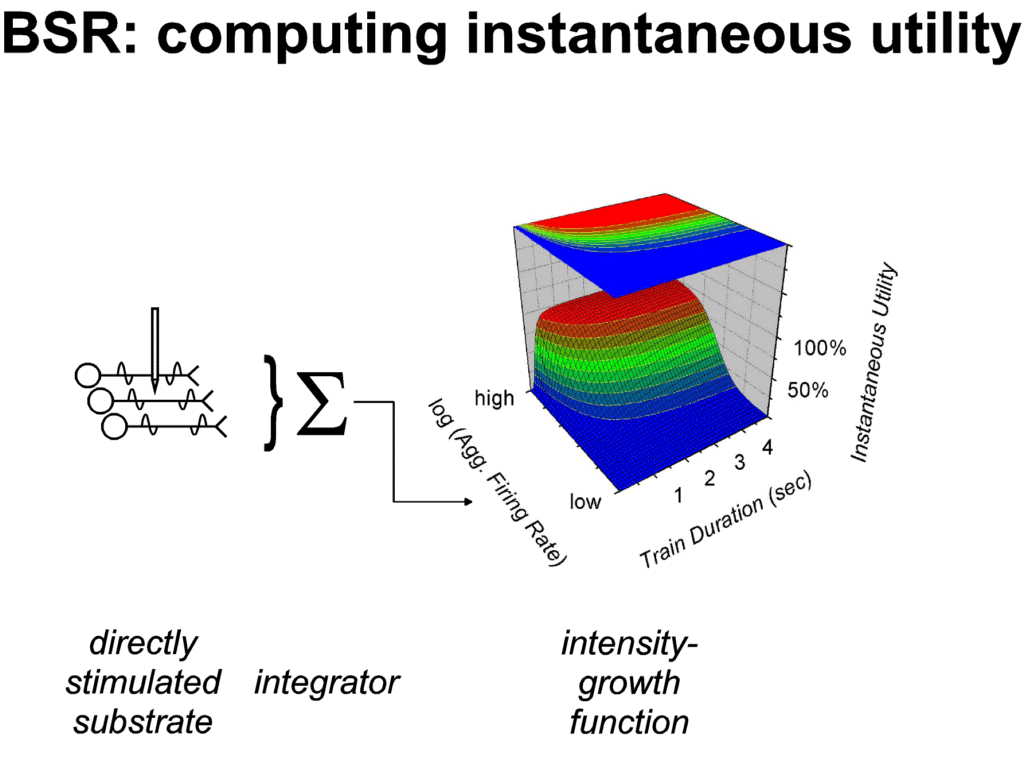 |
| Figure 2: Growth of instantaneous utility as a
function of stimulation strength and duration. The
stronger the stimulation, the higher the aggregate rate
of firing in the directly stimulated neurons responsible
for the rewarding effect. Three relationships are
depicted by the three-dimensional graph. With aggregate
firing rate held constant, instantaneous utility climbs
as the duration of the input is prolonged, eventually
leveling off. This leveling off is responsible for the
"duration neglect" that has been reported in
BSR experiments (Gallistel, 1978; Mark & Gallistel,
1993; Shizgal & Matthews, 1977). With duration held
constant, instantaneous utility climbs steeply as the
aggregate firing rate is increased and then levels off. A
logistic growth function has been used to simulate this
effect. The third relationship is depicted in the
projected contour map. The outlines of successive
horizontal sections through the three-dimensional
structure have been projected onto this plane. Each
contour line gives the combinations of aggregate firing
rate and train duration that raise instantaneous utility
to a given "altitude." The contour lines follow
the hyperbolic form first described by Gallistel (1978).
Changing the "altitude" at which the
cross-section is taken shifts the curve along the axis
representing the logarithm of the firing rate but does
not change the curvature. Plotting the growth of
instantaneous utility as a function of both aggregate
firing rate and train duration illustrates an important
consequence of the parallelism of the contour lines: the
rate at which instantaneous utility grows with train
duration increases as a function of aggregate firing
rate. At high aggregate firing rates, instantaneous
utility approaches asymptote very quickly; at low firing
rates, much more time is required for instantaneous
utility to level off. Results consistent with this
relationship have been reported by Mason and Milner
(1986). |
Duration neglect has also been observed in the case of BSR
(Gallistel, 1978; Mark & Gallistel, 1993; Shizgal &
Matthews, 1977). Based on available data, Figure 2 depicts the
simulated growth of instantaneous utility as a stimulation train
is prolonged. The x-axis of the three-dimensional graph
represents the aggregate firing rate produced by the stimulation
in the neurons responsible for the rewarding effect; the higher
the current or the frequency, the higher the aggregate firing
rate. At each firing rate, the level of instantaneous utility
climbs as the duration of the train is increased, eventually
approaching asymptote. This saturation occurs quickly at high
firing rates and more slowly at low ones (Mason & Milner,
1986). Duration neglect would be manifested by indifferent choice
between two trains of the same strength (trains that produce the
same aggregate firing rate) but different durations. This would
be the case for values lying on the "plateau" of the
depicted surface. Indeed, if the output of one and the same
integrator were responsible both for the instantaneous and
remembered utility of the stimulation, then the surface in figure
2 would not only describe the contribution of aggregate firing
rate and duration to measures of choice, but also to measures of
the resistance to the interruption of ongoing stimulation.
The translation of the instantaneous value of the
stimulation-induced signal into a remembered utility has been
modeled previously as the recording of the peak value (measuring
the height of the plateau in Figure 2) (Gallistel, 1978;
Gallistel et al., 1981). However, Kahneman’s peak-and-end
model makes the same prediction as a peak model in response to a
steady, prolonged input (because the peak height is the same as
the height at the end). Thus, further work is required to see
which model works best in the case of BSR. Indeed, whereas the
instantaneous utility at the end of an experience may be
particularly important in assessing aversive states that one
wishes to terminate, the value at the beginning may have a large
bearing on the assessment of states that one wishes to initiate.
Regardless of the relative contributions of beginnings and ends,
the available data do suggest that the decision utility of BSR is
computed in the spirit of the proposal by Kahneman et al. Rather
than computing the temporal integral of instantaneous utility,
the rat seems to apply a simple rule to a single exemplar value
or a limited set thereof, thus showing profound neglect of
duration. Determining the values that serve as the exemplars of
instantaneous utility during different states and the rules used
to combine these exemplars are important goals for future
research.
In Figure 2, the signal responsible for BSR is portrayed as a
unidimensional quantity that fluctuates in intensity over time as
a function of aggregate firing rate and duration. In the
following sections, the notion that a unidimensional signal is
responsible for BSR is developed and the relationship of this
signal to gustatory reward and the evaluative system are
discussed.
Relationship
between the utility of BSR and gustatory stimuli
A currency function expresses the value of different inputs on
a common scale. Animals behave as if they routinely compute
currency functions because they make orderly choices between
complex, mutually exclusive alternatives, such as returning to
the shelter of a nest or visiting a habitual foraging site. Each
of these alternatives has multiple attributes germane to
physiological regulation and risk. For example, these two options
differ in the probability of finding food and water, losing body
heat, and encountering a predator. Choosing the more valuable
option requires that the multidimensional representations be
"boiled down" to a single common dimension (McFarland
& Sibley, 1975), a common scale of utility.
In the experiments to be reviewed, the choices made by rats
offered various reinforcers were recorded. By definition, these
choices reflect the decision utilities of the available outcomes.
In a later section, I will discuss the translation of remembered
utility into decision utility. For now, let us assume that
remembered utility was the only determinant of decision utility
that varied in the experiments to be reviewed and hence, we can
"see through" the translation process. Given this
assumption and the portrayal provided above of how remembered
utility is computed from instantaneous utility, the observed
choices of the rats can be seen to reflect underlying changes in
instantaneous utility.
To determine whether rats use a common currency to evaluate
rewarding LH stimulation and a sucrose solution, Conover and I
performed two types of experiments. First, we placed the
rewarding LH stimulation in competition with the sucrose by
presenting our subjects with a forced choice between them
(Conover & Shizgal, 1994a). The strength of the BSR was
varied across trials. Not surprisingly, the rats chose the
sucrose in preference to the BSR when the strength of the
electrical stimulation was below the threshold required to
support responding in the absence of the sucrose. When the
strength of the LH stimulation was set somewhat above this
threshold, the rats continued to prefer the sucrose. In other
words, the presence of the sucrose caused the rats to forgo
trains of BSR for which they had worked rather vigorously in the
absence of the gustatory stimulus. However, once the electrical
stimulation was sufficiently strong, BSR was chosen exclusively
in preference to the sucrose. Thus, the rats behaved as if they
had selected the larger of two payoffs evaluated on a common
scale.
In a subsequent experiment, we offered the rats a choice
between BSR alone and a compound reward consisting of an
intraoral infusion of sucrose and an equally preferred train of
BSR. Five of the six rats preferred the compound reward to its
electrical component alone. Thus, the effects of LH stimulation
and sucrose summate in the computation of utility. Summation is
possible only when the inputs share a common property that is
registered by the system of measurement. Given the above
arguments and assumptions, the common property registered by our
system of measurement, behavioral choice, is the ability to drive
instantaneous utility to positive values.
The competition and summation experiments demonstrate that the
LH stimulation and the sucrose have something important in
common, much as Hoebel and others had proposed (Hoebel, 1969).
However, two subsequent experiments demonstrate important
differences between the gustatory and electrical rewards.
In one experiment (Conover, Woodside, & Shizgal, 1994), we
increased the utility of a gustatory stimulus, a sodium chloride
solution, by depleting the subjects of sodium. In the second
experiment (Conover & Shizgal, 1994b), we decreased the
utility of another gustatory stimulus, a sucrose solution, by
allowing large quantities of this solution to accumulate in the
gut. We reasoned that if the LH stimulation recreates the
experience normally produced by a rewarding tastant, then
manipulations that alter the utility of a tastant should have a
similar effect on the BSR. The findings did not support such a
hypothesis. Depleting the rats of sodium by administering a
diuretic dramatically increased the utility of the saline
solution without producing any observable change in the utility
of BSR. Allowing large quantities of a sucrose solution to
accumulate in the gut dramatically reduced the utility of this
solution, in some cases rendering it aversive. However the same
manipulation either failed to alter BSR or produced a much
smaller reduction in the utility of the electrical reward than in
the utility of the gustatory reward.
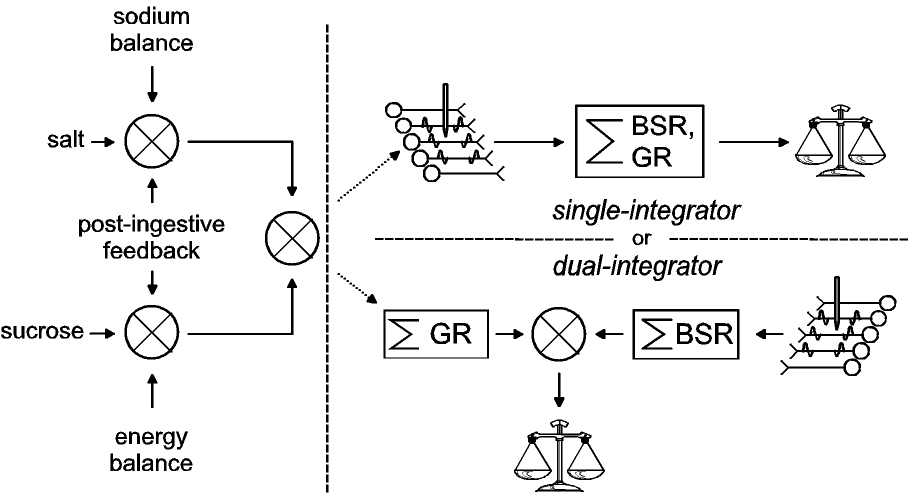 |
| Figure 3: Alternative schemes for combining
the rewarding effects of LH stimulation and gustatory
stimuli. On the basis of experiments by Conover et al.
(Conover & Shizgal, 1994b; Conover et al., 1994),
signals that give rise to gustatory reward are weighted
by physiological feedback prior to their combination with
the signals that give rise to BSR. In the upper right
panel, the two rewards are combined by passing the
gustatory reward through the population of neurons from
which the stimulating electrode samples. Thus, the
post-synaptic effects of the gustatory and electrical
rewards are integrated by a common circuit. In the lower
panel, the gustatory and electrical reward signals are
integrated separately before they are combined and
relayed to the choice mechanism. (Modified from (Shizgal
& Conover, 1996).) |
Our results suggest that although a common signal represents
the instantaneous utilities of the gustatory and electrical
rewards, the LH electrode accesses the neural circuitry that
computes this signal downstream from the point where gustatory
stimuli are weighted by physiological feedback. Two models of how
this could be arranged are shown in Figure 3. Routing
physiological feedback to act at the inputs to the circuitry that
computes the currency, enables behavior to contribute to the
specificity of regulation. This can be seen by considering the
alternative, a system where a currency function returns the
relative utilities of sucrose and saline on a common scale, and
physiological feedback operates uniquely on the output values. In
such a system, changes in sodium balance would alter the utility
of both saline and sucrose solutions as would feedback from the
gut following accumulation of a sucrose load. In contrast, if
physiological feedback weights the inputs to the currency
function, then the relative utilities of saline and sucrose
solutions can be adjusted independently, thus biasing consumption
in response to physiological needs.
Unidimensional
versus multidimensional coding
In typical BSR experiments, neurons are excited within a
relatively large region surrounding the electrode tip (Yeomans,
1990). The argument advanced by Shizgal and Conover (Shizgal
& Conover, 1996) to tie the rewarding effect of BSR to the
output of a currency function addresses the question of how
electrical stimulation of a large population of cells could mimic
a naturally occurring signal. Multiple coding dimensions are
required to capture information about stimulus quality. If only a
single dimension were available, then changes in quality would be
indistinguishable from changes in intensity. This is why we see
the world monochromatically under dim illumination, when only a
single class of photoreceptors is activated. To represent
multiple dimensions of information, some form of spatiotemporal
coding is required. For example, the cells activated by the
stimulus might be divided into multiple sub-populations, each
sensitive to a particular quality ("labeled-line
coding"), or a unitary population might produce different
temporal patterns of activity in response to different stimulus
qualities. In either extreme case or in mixtures thereof, it is
unlikely that gross electrical stimulation would mimic the
multidimensional code. Neurons that do not normally fire in
concert would be activated simultaneously, and all the directly
stimulated cells would fire with the same, rigid,
stimulation-induced periodicity.
In contrast, the electrical stimulation could mimic the effect
of a naturally-occurring stimulus if activity in the stimulated
system represented only a single dimension of information. In
such a system, an aggregate rate code suffices. In such a code,
it matters neither which neuron fires nor when but only how many
firings are produced by the entire population. The spatially
contiguous and temporally synchronous firing evoked by the
electrode could produce the same number of firings in a system
using an aggregate rate code as the spatially discontinuous and
temporally asynchronous firing that is likely evoked by a natural
stimulus. Thus the stimulation-induced activity would mimic the
effect of the natural stimulus. Indeed, in studies of motion
perception in unanesthetized monkeys, micro-stimulation of a
population of neurons that appear to use aggregate firing rate to
code a single perceptual dimension, the direction of visual
motion, can mimic the effect of adding correlated motion to the
elements of a visual stimulus (Newsome & Salzman, 1993).
As discussed above (see "counter model"), there is
strong evidence that the decision utility of BSR and, by
inference, its remembered and instantaneous utility, are derived
from aggregate firing rate. An aggregate code is well-suited to
represent values in a common currency, since, by definition,
these values are arrayed along a single dimension. Thus, values
derived from an aggregate code could be used to compare and
combine the contributions to instantaneous utility of a draught
of sucrose, a draught of saline, or a train of BSR.
The evaluative, perceptual, and timing channels
Inevitably, information is lost in boiling down a
multidimensional representation of a stimulus to obtain a
currency value. For example, one cannot recover the temperature,
sweetness, or texture of a gustatory stimulus from a currency
value representing its instantaneous utility. However, the
information lost due to the collapsing of multiple dimensions is
essential for identifying the stimulus and distinguishing it from
others. Thus the circuitry that computes instantaneous utility
must diverge from the perceptual circuitry subserving
identification and discrimination. This divergence makes it
possible to distinguish between the many different objects and
outcomes that may happen to share the same utility. Similarly, in
order to predict the time when the reinforcer will next be
available, it is important to segregate information about when a
reinforcer was encountered from information in the perceptual and
evaluative channels. Thus, as depicted in Figure 4, information
about reinforcers must be processed in at least three different
ways.
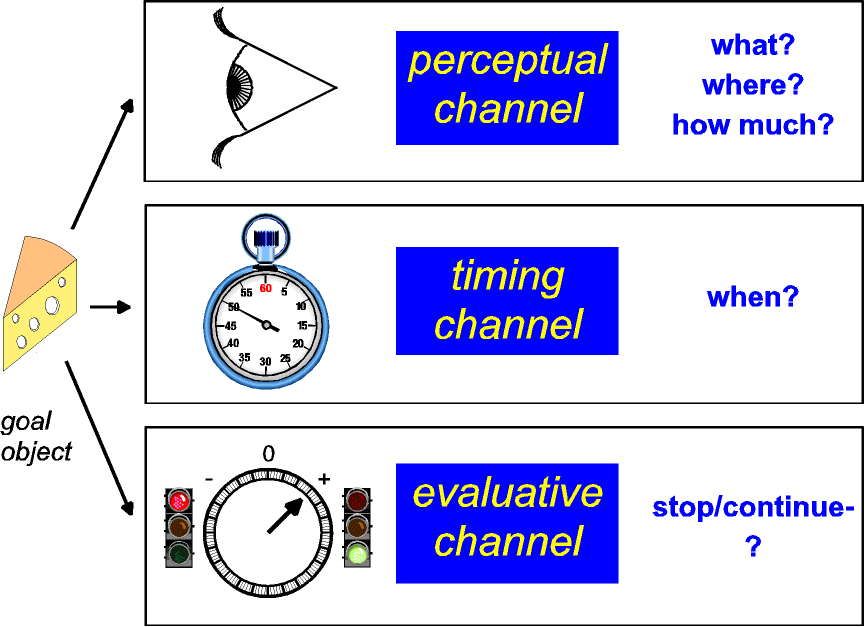 |
| Figure 4: Parallel channels process
information about goal objects in real time. The
perceptual channel returns the identity, location, and
amount of the goal object, whereas a stopwatch-like
channel marks the time when the goal object was
encountered. The evaluative channel steers ongoing
behavior so as to maintain or terminate contact with the
goal object. Given sufficient allocation of attention and
working memory, the output of the evaluative channel may
be manifested in hedonic experience as pleasure or
suffering. |
In this view, the perceptual channel tells the animal what and
where the stimulus is, the evaluative channel returns the
instantaneous utility of the stimulus, and the timer predicts
when the reinforcer will next be available. Gross electrical
stimulation of the evaluative channel could produce a meaningful
signal if, as I have argued, information is encoded in the
stimulated stage by the aggregate rate of firing. In contrast,
gross electrical stimulation is unlikely to produce a meaningful,
multidimensional signal in the perceptual channel because of the
nature of the coding required. What about the response of the
timer? It stands to reason that transitions in the state of many
different channels would be accessible to the timer as
"events." If so, an abrupt change in the activity of
the evaluative channel, such as the stimulation-induced
perturbation responsible for BSR, may provide a sufficient input
to support measurement of temporal intervals.
The perceptual channel is constructed to return facts about
the world. Thus, it is equipped with constancies and
normalization procedures that minimize the impact of changes in
external or internal state on identification and discrimination.
Of course, these constancies and normalization procedures are
imperfect, and bandwidth limitations make it impossible for
perception to be veridical. For example, subjective response
varies non-linearly with changes in the strength of sensory
stimuli, and it is possible to trick the perceptual system into
producing illusions. Nonetheless, the system does a remarkably
good job at estimating objective physical properties such as
size, shape, distance, and reflectance.
The interval timer also appears to be designed to capture data
about objective events. In scalar expectancy theory (Gibbon,
1977), the subjective measure of a temporal interval is a noisy
scalar transform of the objective interval. Although the interval
timer is less accurate than the circadian oscillator, it is
highly flexible, operating over a huge temporal range and
accommodating concurrent timing of multiple intervals with
arbitrary stop and start times (Gibbon, Malapani, Dale, &
Gallistel, 1997).
In contrast to the perceptual and timing channels, the
evaluative channel operates without even a pretense of
objectivity. External objects do not have an inherent worth
independent of present physiological and ecological conditions.
For example, both the sign and magnitude of the instantaneous
utility signal produced by a given stimulus can change as a
function of physiological state (Cabanac, 1971); thus, a cool
stimulus applied to the skin can be refreshing when one is
overheated and unpleasant when one is hypothermic. The evaluative
channel is constructed, not to return objective properties of
stimuli, but rather to return a subjective estimate of the
current significance of these properties.
In contemporary accounts of sensory information processing,
the perceptual "channel" is often treated as a
community of neural modules, each specialized to extract
information of a particular kind such as color, form, movement,
depth and texture. The evaluative channel can also be regarded as
a specialized neural module, charged with the task of deriving
another, more subjective, kind of information from the flow of
sensation. It is not clear whether this module is also composed
of a set of specialized processors, perhaps each linked to a
given modality or combination thereof. If so, the outputs of all
the evaluative processors that influence an ongoing course of
action would have to be combined in order to compute
instantaneous utility. (The continue/stop signal has only one
degree of freedom.) Thus, the output of the evaluative system is
treated here as unitary.
The circuitry responsible for interval timing would appear to
constitute yet another module. Formal models of this
stopwatch-like device have been developed and tested extensively
in behavioral studies. Components of one such model, based on the
scalar expectancy theory of timing (Church, 1984; Gibbon, 1977;
1995), have been linked to the activity of pharmacologically and
anatomically characterized neural populations (Gibbon et al.,
1997; Meck, 1996).
Natural reinforcers are processed by the perceptual,
evaluative, and timing channels. In the following sections, the
notion of remembered utility is generalized to reflect this
parallel processing of information about reinforcers, and the
roles of each of these channels in computing different dimensions
of payoff are discussed.
Subjective
dimensions of payoff
According to the view developed here, changes in the strength
of the stimulation delivered in BSR experiments (e.g., changes in
frequency or current) alter the aggregate firing rate of neurons
that give rise to instantaneous utility. As shown in Figure 5, a
stored record of this response to the change in stimulus strength
is derived by applying a heuristic, such as the peak-and-end
rule, to exemplar values of instantaneous utility, such as the
beginning, peak and end. Variables controlling the strength of
natural reinforcers, such as the concentration of a sucrose
solution or the temperature of an air current, are viewed as
acting analogously, with the exception that the impact of these
variable is weighted by physiological state. Kahneman and his
coworkers (Kahneman et al., 1997; Schreiber & Kahneman,
submitted) have used the term "remembered utility" to
refer to the stored record derived from exemplar values of
instantaneous utility. In the remaining discussion, I will
substitute the term "subjective intensity of the
payoff" for remembered utility in labeling the stored
appraisal of the variables that contribute to stimulus strength.
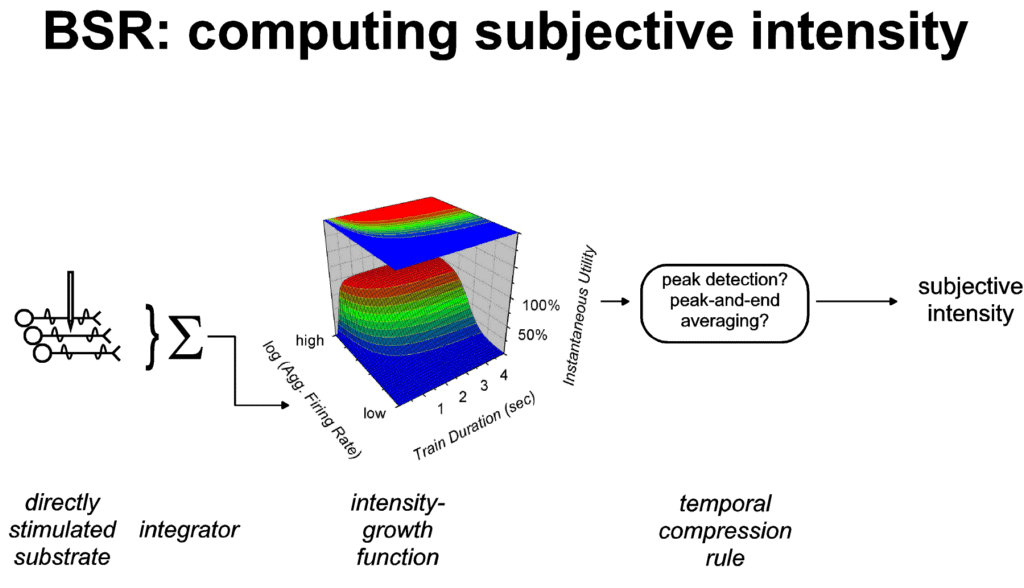 |
| Figure 5: "Representation by
exemplar" (Schreiber & Kahneman, submitted) in
computing the subjective intensity of BSR. The quantity
recorded in memory (the subjective intensity) is derived
from exemplar values of instantaneous utility, such as
the peak (Gallistel, 1978; Gallistel et al., 1981) or the
peak and end (Kahneman et al., 1993; Redelmeier &
Kahneman, 1996). These exemplar values are independent of
the temporal integral of instantaneous utility. Thus,
subjects working for BSR manifest duration neglect: once
instantaneous utility has reached the plateau of the
plotted surface, further increases in duration fail to
increase the remembered subjective intensity. |
Why introduce yet another term? I do this because decision
utilities reflect not only the output of the evaluative channel
subserving instantaneous utility but also the outputs of the
timing and perceptual channels. Thus, the effects of reinforcers
on future choices depend not only on their strength, but also on
their rate, delay, amount, and kind. The notion of remembered
utility proposed by Kahneman, Wakker, and Sarin (1997) and
developed by Schreiber and Kahneman (submitted) is tied to the
intensity dimension alone. A more general means of describing
recorded payoffs is required if we are to capture the
multidimensional contribution of reinforcers to decision utility.
To illustrate the need for a multidimensional treatment of
reinforcement, consider the interaction of the rate and strength
of reinforcement. Via preference tests, it can be shown that rats
prefer highly concentrated solutions to less concentrated ones
(Young, 1967). This difference in the intensity of the payoff can
be offset by a compensatory change in its rate (Heyman &
Monaghan, 1994): the allocation of time or responding to the two
solutions can be equated by making the less concentrated solution
available more frequently than the highly concentrated one. The
same relationship between stimulus strength and rate of
reinforcement in determining behavioral allocation seems to hold
when BSR, rather than a natural goal object, serves as the
reinforcer (Hamilton, Stellar, & Hart, 1985). Similarly,
weaker trains of stimulation are preferred equally to stronger
trains when the rate at which the weaker trains are available is
sufficiently high (Gallistel, 1991).
That both the rate and strength of reinforcers contribute to
payoff suggests that the stored record of the reinforcer is
multidimensional. The perceptual, timing, and evaluative channels
not only process information about reinforcers in parallel, they
also record their outputs in parallel. Payoff is then computed by
combining the contents of the multidimensional record. In this
view, illustrated in Figure 6, subjective intensity is the
dimension of the stored record derived from the output of the
evaluative channel. An output of the timing channel constitutes
the second dimension. The nature of this stored quantity is a
matter of debate. In one well-formulated proposal, this temporal
dimension of the stored record contains a noisy measure of the
inter-reinforcer interval (the inverse of reinforcement rate)
(Gibbon, 1995). For convenience of phrasing I will use the term
"subjective rate of payoff" to refer to this dimension
of the stored record, leaving open the possibility that the
stored quantity is not a rate per se but rather a measure
from which a rate could be derived, such as an
inter-reinforcement interval.
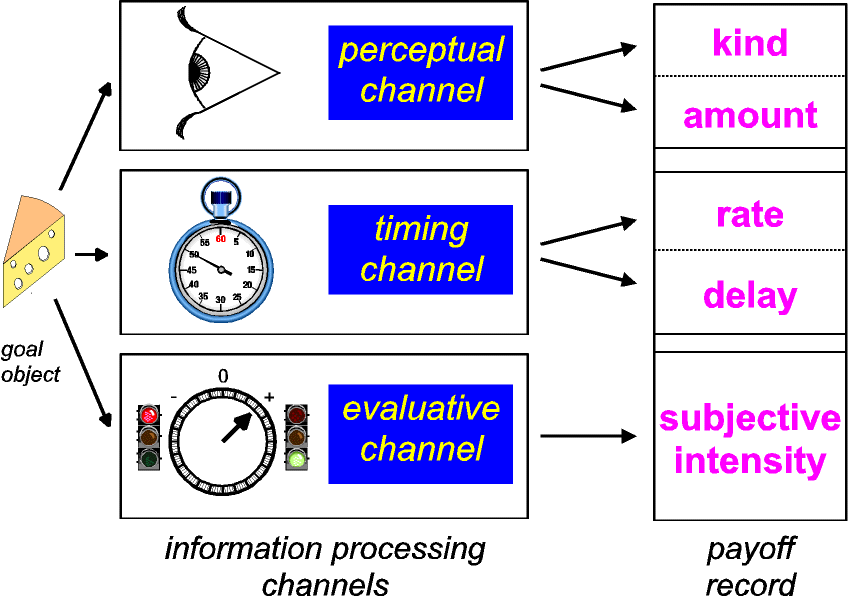 |
| Figure 6: Recording the output of the parallel
information-processing channels. Stored information from
all three channels contributes to payoff. Information
derived from the perceptual channel indicates
"kind" (is the goal object a source of food,
water, or salt?) as well as amount. Estimates of the
encounter rate and the delay between a successful
response and delivery of a reinforcer are derived from
the output of the stopwatch timer. The evaluative channel
contributes an estimate of subjective intensity (see Fig.
4) to the payoff record. |
In addition to subjective rate, the timer provides another
quantity that contributes to subjective payoff: the delay between
the reinforced response and the delivery of the reinforcer.
Payoff appears to decline hyperbolically as the presentation of
the reinforcer is delayed (Commons, Mazur, Nevin, & Rachlin,
1987; Mazur, 1986; Myerson & Green, 1995). This relationship
appears to hold for BSR as well as for natural reinforcers
(Mazur, Stellar, & Waraczynski, 1987).
The treatment of BSR presented here implies that a record
consisting of a subjective intensity and the subjective weighting
of rate and delay is sufficient for computing a payoff on which a
decision utility can be based. However, in the case of natural
stimuli, additional dimensions contribute to payoff. For example,
reinforcers, such as food pellets, may vary in mass. It stands to
reason that the amount of a natural reinforcer would be recovered
from perceptual information such as size and heft, and that
unlike the case of intensity, the estimation of amount would be
stable in the face of changes in physiological state. For
example, one would hope that hunger would not alter one’s
judgments about the size of the fruit in a tree. Thus, an
additional dimension of remembered payoff, the subjective
weighting of amount, is likely returned by the perceptual
channel. If BSR is not accompanied by a meaningful signal in the
perceptual channel, then the information in this cell of the
payoff record is likely to be absent or indecipherable.
The contribution of payoff to decision utility would appear to
involve at least two stages of processing. First, a stored record
is obtained by mapping physical dimensions such as strength,
rate, delay, and amount into corresponding subjective ones.
Decision utility would appear to reflect the result of performing
a combinatorial operation on the quantities in the stored record
of payoff. Accounts of matching, to be discussed in the next
section, tend to treat the combinatorial operation in question as
multiplication (Baum & Rachlin, 1969; Davison & McCarthy,
1988). Figure 7 depicts multiplicative combination of intensity
and rate in computing the subjective payoff provided by a train
of rewarding stimulation.
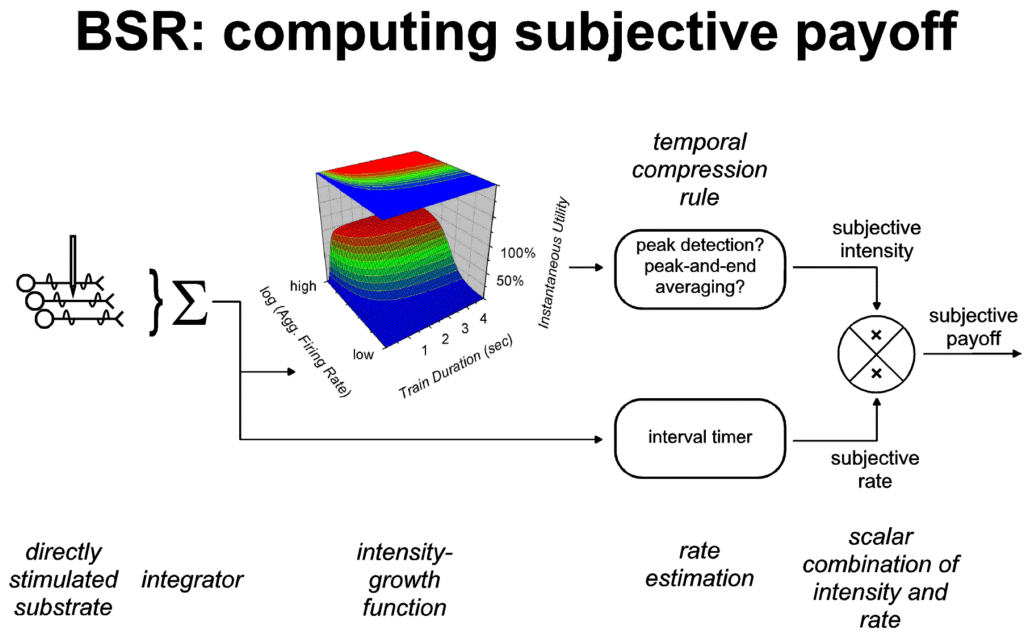 |
| Figure 7: Computation of the subjective payoff
provided by a train of rewarding stimulation. The
left-hand portion of the figure, reproduced from Figures
1 and 2, shows how the instantaneous utility of the
rewarding stimulation is derived from the aggregate
firing rate in the directly stimulated stage of the
underlying neural circuit. Via the principle of
representation by exemplar, instantaneous utility is
transformed into the subjective intensity of the payoff,
one of the dimensions of the stored record of subjective
payoff. Two possible rules for carrying out this
transformation are shown: peak-and-end averaging and peak
detection. A second dimension of the stored record, the
subjective rate of payoff, is provided by an interval
timer. On the basis of research on operant matching, the
combinatorial operation for combining these two
dimensions is shown as multiplication. |
Matching:
translation of subjective payoff into decision utility
Imagine a pair of exquisite but idiosyncratically managed
restaurants. The quality of the cuisine may be truly outstanding,
and the philanthropic proprietors demand no remuneration other
than the commitment of time by the diners. Each restaurant is
open a certain number of times per month on the average, a rate
that may or may not differ from the accessibility of the
competing establishment. Although the average rate at which each
restaurant opens is constant over time, the interval between
openings varies randomly. Openings are unannounced, thus keeping
the clientele guessing as to the date and time when they can gain
entry. Sometimes, a client arrives to find the chosen restaurant
already open; on other occasions, the restaurant is closed at the
time of arrival, and the client may either wait until the
restaurant opens or leave beforehand. Once a seat at a table has
been secured, the diners face a delay until the serving of the
first course that may differ in the two establishments,. Finally,
both chefs are experimenting with different portion sizes;
currently, one leans towards "cuisine minceure" and the
other towards the fashion of a Chicago steak house.
The matching law was formulated to describe the allocation of
behavior in experimental settings roughly analogous to the
competing restaurants. Lest the reader find the capricious
scheduling too bizarre to take seriously, I should point out that
the unpredictable availability of a reinforcer might well seem
more realistic to people living in the manner of our ancestors.
The traditional Inuit hunter did not expect a seal to visit a
particular breathing hole in the ice at any designated time, yet
he derived an estimate of the average frequency of visits and
allocated his time accordingly.
In the terminology of operant conditioning, the diners in the
above example are presented with concurrent variable-interval
schedules of reinforcement According to the strict form of the
matching law (Davison & McCarthy, 1988; de Villiers, 1977;
Herrnstein, 1961; Herrnstein, 1970; Williams, 1988), they will
allocate their time and visits in proportion to the relative
payoffs provided by the two restaurants. These payoffs are
calculated by multiplicative combination of the subjective
intensity (the "goodness" of the food) with the
subjective weightings of the rate of opening, delay of meal onset
and portion size. In the terms employed here, the matching law
translates the multidimensional records of subjective payoffs
into decision utilities. Under the strict form of the matching
law, relative decision utility is proportional to relative
subjective payoff.
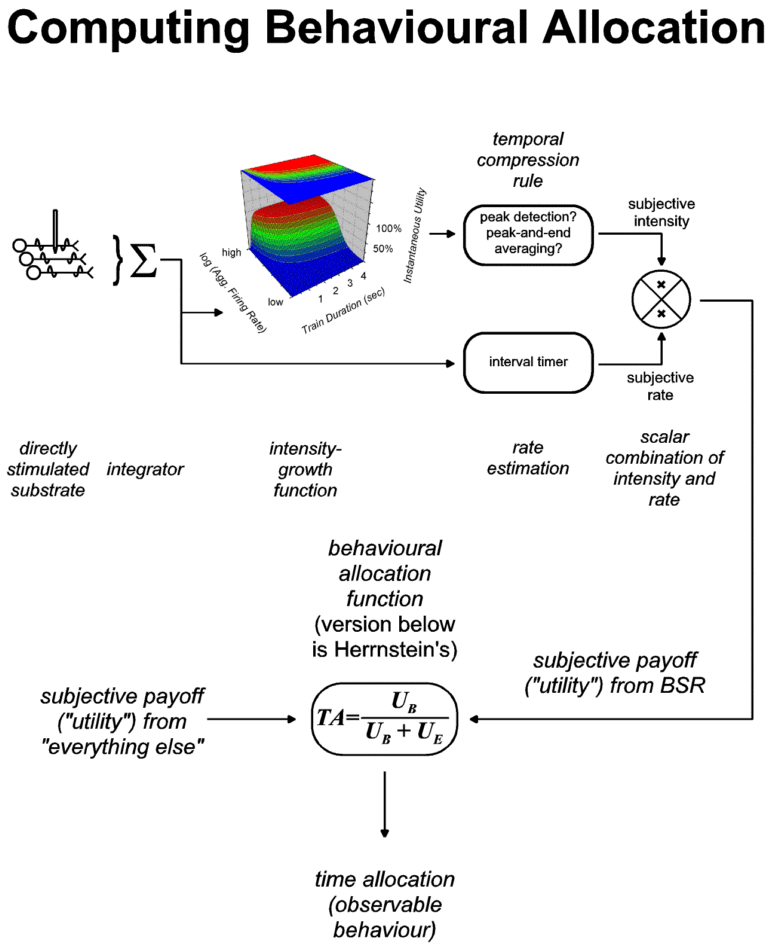 |
Figure 8. Translation of subjective payoff
into behavior according to Herrnstein’s treatment of
operant performance for a single experimenter-controlled
reinforcer. According to Herrnstein’s view, the
payoff obtained by working for the
experimenter-controlled reinforcer is compared to the
payoff from competing activities such as grooming,
exploring, and resting ("everything else"). The
allocation of behavior to the experimenter-controlled
reinforcer is determined by the payoff it provides as a
proportion of the sum of all payoffs available in the
test environment. This view of behavioral allocation runs
into difficulty when the subject works for an essential
natural reinforcer unavailable outside the test
environment or when the subject chooses between two
natural reinforcers of different kinds. However, neither
of these restrictions apply in the case of BSR. Reasons
why performance for BSR and for natural reinforcers
differ in these respects are discussed in the text. |
The subject performing on concurrent variable-interval
schedules can be portrayed as repeatedly flipping a biased coin,
with the bias reflecting the relative payoffs provided by the two
reinforcers (Gibbon, 1995; Heyman, 1988; Heyman & Goodman,
submitted). If so, the relative payoffs will be reflected in the
relative allocations of time to the two schedules. Given strict
matching and multiplicative combination of subjective intensity
and rate, the ratio of the subjective intensities of two
reinforcers can then be calculated from the observed ratios of
reinforcement rates and time allocation. This logic was used by
Miller (1976) to measure, in pigeons, the relative intensity of
the payoffs provided by three different types of seeds and by
Gallistel and his students (Gallistel, 1991; Leon &
Gallistel, 1992; Simmons & Gallistel, 1994) to measure how
the subjective intensity of BSR grows as a function of the
strength of electrical stimulation in rats. Gallistel’s
group found that as the stimulation strength rises above
threshold, the subjective intensity of the payoff climbs steeply,
initially approximating a power function; the growth eventually
slows and levels off as stimulation strength is increased to ever
higher levels. In Figures 2, 5, 7 and 8, the growth of BSR as a
function of the aggregate rate of stimulation-induced firing is
modeled as a logistic, thus capturing the steep initial rise, the
later deceleration, and the eventual leveling off.
Knowing the form and parameters of the intensity-growth
function could serve as a powerful constraint in interpreting
recordings of neural activity. For example, if one wished to
argue that a particular population of neurons encodes the
intensity of the payoff produced by the rewarding stimulation,
one would have to demonstrate that some attribute of activity in
this population corresponds to the form and parameters of the
intensity-growth function.
Beyond
strict matching: contribution of economic constraints to decision
utility
The above discussion of matching was confined to cases in
which the two competing reinforcers are of the same kind (e.g.,
food) and where the reinforcers are available outside the test
environment. When reinforcers of different kinds are pitted
against each other in choice experiments or when a natural
reinforcer is available uniquely in the test environment, the
strict form of the matching law may no longer account gracefully
for the translation of subjective payoffs into decision utility.
Two additional pieces of information appear to contribute to the
computation of decision utility: the category to which the
reinforcer belongs (its kind) and the environmental distribution
of the reinforcer. The role of the perceptual channel in
providing this information is highlighted by experiments in which
BSR competes with natural reinforcers.
The needs addressed by behavioral means are many and varied.
Thus, humans and other animals seek "goods" of
different kinds. If a sufficiently broad time frame is adopted,
choices between alternatives are rarely constrained to single
decisions between mutually exclusive options. Under such
circumstances, the problem of adaptive choice is to select the
best "bundle" of goods rather than the single item with
the highest value in a common currency. Research in behavioral
economics suggests that when available goods are not of the same
kind, calculating the utility of the bundle will usually require
an operation more complex than simply summing the utilities of
the individual items (Kagel, Battalio, & Green, 1995;
Rachlin, Green, Kagel, & Battalio, 1976; Rachlin, Kagel,
& Battalio, 1980).
The relationships between the items in the bundle can be
arrayed on a continuum. At one extreme are goods that are
entirely substitutable, like two brands of cola to an indifferent
consumer. At the other extreme are goods that are complements,
such as bicycle frames and bicycle wheels or left shoes and right
shoes. In the case of perfect substitutes, the utility of the
bundle is simply the sum of the utilities of the constituent
goods. In contrast, the more complementary the constituents, the
less each is worth individually in comparison with the utility of
the bundle.
The distinction between substitutes and complements has
profound behavioral implications. For example, if the relative
prices of two highly substitutable goods are changed and the
budget of the consumer is adjusted so as to make possible the
purchase of the same quantities as had been acquired at the
former prices, consumption shifts toward the cheaper good. In
contrast, complementary goods tend to be consumed in a fixed
ratio, which is insensitive to changes in relative price.
When substitutability is taken into account, the simple form
of the matching law no longer suffices. For example, responding
for the reinforcer on the richer of two schedules will increase
when the schedule for a perfect substitute is made leaner
(increasing its "price"), as the matching law predicts.
However, responding on the richer schedule will decrease when the
schedule for a perfect complement is made leaner. Rachlin, Kagel
and Battalio (Rachlin et al., 1980) have shown how a
generalization of the simple form of the matching law can be
interpreted to incorporate substitutability. Each of the ratios
reflecting the subjective weighting of rate, delay, amount, and
strength is first raised to an exponent before scalar
combination. For perfect substitutes, the exponent is one, and
the equation reduces to the simple form of the matching law.
According to this modification to the matching law, the
translation of subjective payoff into decision utility depends on
the substitutability of the reinforcers.
Substitutability of BSR
and natural reinforcers. Results of an experiment carried out
by Green and Rachlin to measure the substitutability of rewarding
LH stimulation, food, and water (Green & Rachlin, 1991) can
be interpreted in terms of the role of the perceptual channel in
computing decision utility. As expected, they found that food and
water were poor substitutes. Nonetheless, BSR was highly
substitutable for both food and water.
Presumably, dry food and water are poor substitutes because
they fulfill non-overlapping physiological needs. If so, does the
high substitutability of BSR with both food and water imply that
the signal injected by the electrode mimics signals specific both
to energy balance and fluid balance? If this were the case, one
would expect that BSR would be less substitutable for food and
water than for itself. This isn’t what Green and Rachlin
found. BSR triggered by one lever was about as substitutable for
BSR triggered by the other lever as for food or water.
One interpretation suggested by Green and Rachlin is that BSR
acts as a "general" reinforcer. This is reminiscent of
the coding argument advanced by Shizgal and Conover (Shizgal
& Conover, 1996). We proposed that any activity produced by
the rewarding stimulation in the perceptual system would tend to
be "noisy" and would be unlikely to mimic the signature
of a naturally occurring goal object. Moreover, our results
suggest that the rewarding stimulation acts downstream from the
point where physiological feedback weights gustatory rewards.
Thus, there may well be no way for the rat to determine the
regulatory system or the class of goal object to which the
stimulation is germane. However, our data suggest that the
evaluative channel produces a unidimensional currency signal in
response to rewarding brain stimulation, a sucrose solution and a
saline solution. If this were also the case for water and for the
food employed by Green and Rachlin, then these goods would also
substitute well for BSR. In contrast, food and water would
register distinct signals in the perceptual channel, making it
possible to incorporate the identity, and hence, the low
substitutability, of these two reinforcers when computing the
relative decision utilities of the activities that produce these
two goods.
Elasticity of demand for
BSR and natural reinforcers. Most experiments that employ
food reinforcement are conducted under the conditions of an
"open economy:" food is made available during the
relatively short test sessions and is also available during at
least some of the much longer period the subject spends in its
home cage. Often, the body weight of the subjects is maintained
at some proportion of the free-feeding value. Thus, the
supplementary food given to the subjects in the home environment
can bring total intake to a fixed level when added to what was
earned in the test environment. In such circumstances,
"demand" (the number of reinforcers earned in the test
environment) is said to be highly "elastic," changing
steeply in response to variations in "price" (the
number of responses required to obtain a single reinforcement).
For example, the higher the cost of food in the experimental
situation, the less food the animal earns there (Hursh, 1980). In
contrast, when the economy is "closed," and the
reinforcer is available uniquely in the test environment, demand
for food becomes highly inelastic over an appreciable
range of prices (Collier, Johnson, Hill, & Kaufman, 1986;
Hursh, 1980). When the subject must fend for itself, without the
benefit of supplements, response rates tend to increase with
price so that consumption is largely defended.
Although it is tempting to attribute the inelastic demand
observed in a closed economy to an accumulating effect of
deprivation, the data do not fit this hypothesis gracefully. Over
the range of prices within which demand remains highly inelastic
in such experiments, subjects can maintain their body weight or
even increase it (Collier, 1983). If so, some aspect of the
economic circumstances appears to be altering the translation of
subjective payoff into decision utility.
According to the single-operant version of the matching law
(Herrnstein, 1970), subjects presented with a single
experimenter-supplied reinforcer choose between working for it
and performing alternative activities, such as the opportunity to
groom, rest, or explore. Herrnstein’s formulation predicts
that increasing the price of the experimenter-supplied reinforcer
will shift the allocation of behavior away from that reinforcer
and towards "everything else." The effect of price on
performance for food in a closed economy violates this form of
the matching law: contrary to prediction, relative allocation of
behavior to natural reinforcers is no longer proportional to
relative payoff.
The situation is different in the case of BSR. In the great
majority of BSR experiments, the economy is closed; no
stimulation is available in the home cage. Yet demand for BSR is
highly elastic; the larger the number of responses required to
earn a reinforcement or the more stringent the limit on maximum
consumption per unit time, the lower the allocation of behavior
to responding for BSR (Druhan, Levy, & Shizgal, 1993;
Fouriezos, Emdin, & Beaudoin, 1996; Hamilton et al., 1985).
Thus, as Hursh and Natelson (1981) have demonstrated, closing the
economy produces very different changes in performance for BSR
and food: demand for BSR remains highly elastic whereas demand
for food is highly inelastic.
The effect of closing the economy on responding for food and
water reinforcement has been interpreted to suggest that the
subject learns the relative availability of the reinforcer in the
different environments to which it is exposed (Collier, 1983),
much as optimal foraging theorists have postulated (Charnov,
1976; Stephens & Krebs, 1986). Such a representation would
appear to store information from the perceptual channel in a
spatial or spatiotemporal context (e.g., food is available in the
test chamber, but not in the home cage) and to contribute, along
with the subjective weightings of rate, delay, amount, and
strength, to determining decision utility. The situation for BSR
seems far simpler: demand for BSR appears unaffected by whether
or not the economy is open (although direct experimental
confirmation of this prediction is lacking). Perhaps this is so
because the "noisy" signal produced by rewarding brain
stimulation in the perceptual channel cannot be attributed to a
particular natural reinforcer.
Expectancy
In the fanciful example described above, the prospective
diners could not predict the time when either of the restaurants
would next open. Although the availability of resources in the
natural world may also be unpredictable, there are circumstances
in which events occur in reliable sequences. For example, after a
flower has been drained by a hummingbird, the nectar will tend to
accumulate at a characteristic rate, and the bird will adjust the
timing of its return accordingly (Gallistel, 1990). Subjects
working on fixed-interval schedules of reinforcement in
laboratory experiments manifest a pause in responding after
delivery of the reinforcer; responding then resumes as the time
to the next scheduled delivery of reinforcement approaches
(Gibbon, 1977). In such circumstances, decision utility is
adjusted dynamically to reflect information provided by the
interval timer concerning the scheduling of reinforcement. The
probability that the animal will direct its behavior towards the
goal shifts from low to high as the predicted time of
reinforcement approaches. This adjustment in behavior is
anticipatory and thus, the animal can be said to have formed an
expectation of future payoff.
In scalar expectancy theory, the average level of expectancy
is increased by a large recent payoff (Gibbon, 1977). It stands
to reason that this increase might be specific to the expectancy
of future payoffs of the same kind as the large, recent one. This
proposal is in the spirit of Loewenstein’s (1996) treatment
of the focusing effects produced by sensations and states that
have large instantaneous utilities . Such sensations and states
are portrayed as "crowding out" consideration of
alternate reinforcers.
With this view of expectancy in mind, let us return to the
vignette at the beginning of this chapter. Towards the end of the
rat’s confinement in the start box, it approaches the
barrier blocking access to the alley. Such behavior is much more
pronounced on trials when stimulation is delivered in the start
box than on trials when it is not; on the trials preceded by
stimulation, the rat not only approaches the barrier but makes
frenzied attempts to climb over it. This potentiation of
anticipatory acts cannot be due simply to stimulation-induced
arousal because the same start-box stimulation fails to galvanize
behavior when the rat has learned that additional stimulation is
no longer available in the goal box. Rather, the experience of a
large, recent payoff seems to rescale expectancy (Sax &
Gallistel, 1990), boosting its magnitude at each point in time as
the delay to accessing the alley elapses. However, when the rat
has learned that reinforcement is no longer available in the goal
box, there is, in effect, no expectancy for the start-box
stimulation to rescale, and the rat’s behavior in the start
box becomes nonchalant.
The effect of a large, recent payoff on decision utility is
illustrated by two experiments in which thirsty rats chose
between BSR and water (Deutsch, Adams, & Metzner, 1964;
Wasserman, Gomita, & Gallistel, 1982). Shortly following
delivery of pretrial stimulation, the rats chose BSR in
preference to water. When the pretrial stimulation was omitted,
the preference reversed, and the rats opted for the water instead
of the BSR. The pretrial stimulation was very similar to the
stimulation offered as a reward. In contrast, the stimulation is
unlikely to have mimicked the perceptual experience produced by
the water (see "Unidimensional versus multidimensional
coding"). According to the argument sketched out above, the
expectancy boosted by the large pretrial payoff will be directed
at the reward with the neural signature most similar to that of
the pretrial stimulation. This augmented expectancy would boost
the decision utility of BSR, thus biasing choice. It would be
interesting to determine whether pretrial exposure to water would
produce a complementary effect. More generally, the dependence of
decision utility on the interaction between the experience,
expectation, and recording of payoffs deserves additional
attention. Rewarding brain stimulation may prove to be a valuable
tool for investigating such interactions.
Coda
Vigorous efforts are underway to identify the components of
the neural circuitry responsible for BSR. Although the quarry has
long proved elusive, new methods for visualizing candidate
neurons (Arvanitogiannis, Flores, Pfaus, & Shizgal, 1996a;
Arvanitogiannis, Flores, & Shizgal, 1997; Flores,
Arvanitogiannis, & Shizgal, 1997) and for measuring
behavioral effects of drugs and lesions (Arvanitogiannis,
Waraczynski, & Shizgal, 1996b; Shizgal, Conover, &
Arvanitogiannis, 1996) offer hope for better hunting. Once these
cells are found, we will have new questions to ask them as a
result of this attempt to align conceptions of utility that have
grown out of the study of BSR and natural reinforcers in
laboratory animals with ideas derived from the study of
evaluation and choice in humans. It will be particularly
interesting to test the hypothesis that the neurons responsible
for the signal recorded as the subjective intensity of the payoff
also give rise to the instantaneous utility of the stimulation.
By recording from these neurons in awake, behaving subjects, it
should prove possible to test many of the hypotheses discussed
here concerning the role of these cells in the computation of
utility. Among these hypotheses is the proposal that the output
of the directly activated neurons underlying BSR can gain access
to working memory under attentional control. Investigating this
hypothesis could shed light on how signals fundamental to the
experience of pleasure gain access to awareness.
References
Arvanitogiannis, A., Flores, C., Pfaus, J. G., & Shizgal,
P. (1996a). Increased ipsilateral expression of Fos following
lateral hypothalamic self-stimulation. Brain Research, 720,
148-154.
Arvanitogiannis, A., Flores, C., & Shizgal, P. (1997).
Fos-like immunoreactivity in the caudal diencephalon and
brainstem following lateral hypothalamic self-stimulation. Behavioural
Brain Research, 88(2), 275-279.
Arvanitogiannis, A., Waraczynski, M., & Shizgal, P.
(1996b). Effects of excitotoxic lesions of the basal forebrain on
MFB self-stimulation. Physiology & Behavior, 59(4/5),
795-806.
Baars, B. J. (1988). A Cognitive theory of consciousness.
Cambridge: Cambridge University Press.
Baum, W. M., & Rachlin, H. (1969). Choice as time
allocation. Journal of the Experimental Analysis of Behavior, 12,
861-874.
Bentham, J. (1996 (originally published: 1789)). An
introduction to the principles of morals and legislation.
Oxford: Clarendon Press.
Bielajew, C., & Shizgal, P. (1980). Dissociation of the
substrates for medial forebrain bundle self-stimulation and
stimulation-escape using a two-electrode stimulation technique. Physiology
and Behavior, 25, 707-711.
Bishop, M. P., Elder, S. T., & Heath, R. G. (1963).
Intracranial self-stimulation in man. Science, 140,
394-6.
Bower, G. H., & Miller, N. E. (1958). Rewarding and
punishing effects from stimulating the same place in the rat's
brain. Journal of Comparative and Physiological Psychology, 51,
669-674.
Boyd, E. S., & Gardiner, L. C. (1962). Positive and
negative reinforcement from intracranial stimulation of a
teleost. Science, 136, 648-9.
Cabanac, M. (1971). Physiological role of pleasure. Science,
173, 1103-1107.
Cabanac, M. (1992). Pleasure: the common currency. Journal
of Theoretical Biology, 155, 173-200.
Cabanac, M., & LeBlanc, J. (1983). Physiological conflict
in humans: fatigue vs. cold discomfort. American Journal of
Physiology, 244, R621-R628.
Charnov, E. L. (1976). Optimal foraging: the marginal value
theorem. Theoretical Population Biology, 9,
129-136.
Church, R. M. (1984). Properties of the internal clock. In J.
Gibbon & L. Allan (Eds.), Timing and Time Perception
(Vol. 423, pp. 566-582). New York: New York Academy of Sciences.
Collier, G. H. (1983). Life in a closed economy: The ecology
of learning and motivation. In M. D. Zeller & P. Harzem
(Eds.), Advances in Analysis of Behaviour (Vol. 3, pp.
223-274). New York: John Wiley & Sons.
Collier, G. H., Johnson, D. F., Hill, W. L., & Kaufman, L.
W. (1986). The economics of the law of effect. Journal of the
Experimental Analysis of Behavior, 46, 113-136.
Commons, M. L., Mazur, J. E., Nevin, J. A., & Rachlin, H.
(Eds.). (1987). Quantitative Analysis of Behavior: The Effects
of Delay. (Vol. 5). Cambridge, MA: Ballinger.
Conover, K. L., & Shizgal, P. (1994a). Competition and
summation between rewarding effects of sucrose and lateral
hypothalamic stimulation in the rat. Behavioral Neuroscience, 108(3),
537-548.
Conover, K. L., & Shizgal, P. (1994b). Differential
effects of postingestive feedback on the reward value of sucrose
and lateral hypothalamic stimulation in the rat. Behavioral
Neuroscience, 108(3), 559-572.
Conover, K. L., Woodside, B., & Shizgal, P. (1994).
Effects of sodium depletion on competition and summation between
rewarding effects of salt and lateral hypothalamic stimulation in
the rat. Behavioral Neuroscience, 108(3), 549-558.
Davison, M., & McCarthy, D. (1988). The Matching Law.
Hillsdale, NJ: Lawrence Erlbaum Associates.
de Villiers, P. (1977). Choice in concurrent schedules and a
quantitative formulation of the law of effect. In W. K. Honig
& J. E. R. Staddon (Eds.), Handbook of Operant Behavior
(pp. 233-287). Englewood Cliffs, NJ: Prentice Hall.
Deutsch, J. A., Adams, D. W., & Metzner, R. J. (1964).
Choice of intracranial stimulation as a function of delay between
stimulations and strength of competing drive. Journal of
Comparative and Physiological Psychology, 57, 241-243.
Distel, H. (1978). Behavior and electrical brain stimulation
in the green iguana, Iguana iguana L. II. Stimulation effects. Experimental
Brain Research, 31(3), 353-367.
Druhan, J. P., Levy, M., & Shizgal, P. (1993). Effects of
varying reinforcement schedule, reward current, and pretrial
priming stimulation on discrete-trial performance for brain
stimulation reward. Psychobiology, 21(1), 37-42.
Edmonds, D. E., & Gallistel, C. R. (1974). Parametric
analysis of brain stimulation reward in the rat: III. Effect of
performance variables on the reward summation function. Journal
of Comparative and Physiological Psychology, 87,
876-883.
Flores, C., Arvanitogiannis, A., & Shizgal, P. (1997).
Fos-like immunoreactivity in forebrain regions following
self-stimulation of the lateral hypothalamus and the ventral
tegmental area. Behavioural Brain Research, 87(2),
239-251.
Fouriezos, G., Emdin, K., & Beaudoin, L. (1996).
Intermittent rewards raise self-stimulation thresholds. Behavioural
Brain Research, 74, 57-64.
Frank, R. A., & Stutz, R. M. (1984). Self-deprivation: A
review. Psychological Bulletin, 96(2), 384-393.
Fredrickson, B. L., & Kahneman, D. (1993). Duration
neglect in retrospective evaluations of affective episodes. Journal
of Personality and Social Psychology, 65(1), 45-55.
Gallistel, C. R. (1978). Self-stimulation in the rat:
Quantitative characteristics of the reward pathway. Journal of
Comparative and Physiological Psychology, 92, 977-998.
Gallistel, C. R. (1980). The organization of action: a new
synthesis. Hillsdale, New Jersey: Lawrence Erlbaum
Associates.
Gallistel, C. R. (1990). The organization of learning.
Cambridge, Massachusetts: MIT Press.
Gallistel, C. R. (1991). Measuring the subjective magnitude of
brain stimulation reward by titration with rate of reward. Behavioral
Neuroscience, 105(6), 913-925.
Gallistel, C. R., Shizgal, P., & Yeomans, J. S. (1981). A
portrain of the substrait for self-stimulation. Psychological
Review, 88, 228-273.
Gibbon, J. (1977). Scalar expectancy theory and Weber's law in
animal timing. Psychological Review, 84(3),
279-325.
Gibbon, J. (1995). Dynamics of time matching: arousal makes
better seem worse. Psychonomic Bulletin & Review, 2(2),
208-215.
Gibbon, J., Church, R. M., Fairhurst, S., & Kacelnik, A.
(1988). Scalar expectancy theory and choice between delayed
rewards. Psychological Review, 95(1), 102-114.
Gibbon, J., Malapani, C., Dale, C. L., & Gallistel, C.
(1997). Towards a neurobiology of temporal cognition: advances
and challenges. Current Opinion in Neurobiology, 7(2),
170-184.
Goldman-Rakic, P. S. (1996). Regional and cellular
fractionation of working memory. Proceedings of the National
Academy of Sciences, USA, 93, 13473-13480.
Green, L., & Rachlin, H. (1991). Economic substitutablity
of electrical brain stimulation, food, and water. Journal of
the Experimental Analysis of Behavior, 55, 133-143.
Hamilton, A. L., Stellar, J. R., & Hart, E. B. (1985).
Reward, performance, and the response strength method in
self-stimulating rats: validation and neuroleptics. Physiology
& Behavior, 35, 897-904.
Heath, R. G. (1964). Pleasure response of human subjects to
direct stimulation of the brain: Physiologic and psychodynamic
considerations. In R. G. Heath (Ed.), The Role of Pleasure in
Behavior (pp. 219-243). New York: Harper and Row.
Heller, J. (1961). Catch-22. New York: Simon &
Schuster.
Herrnstein, R. J. (1961). Relative and absolute strength of
response as a function of frequency of reinforcement. Journal
of the Experimental Analysis of Behavior, 4, 267-272.
Herrnstein, R. J. (1970). On the law of effect. Journal of
the Experimental Analysis of Behavior, 13(2), 243-266.
Heyman, G. (1988). How drugs affect cells and reinforcement
affects behavior: formal analogies. In M. L. Commons, R. M.
Church, J. R. Stellar, & A. R. Wagner (Eds.), Biological
Determinants of Reinforcement (Vol. VII, pp. 157-182).
Hillsdale, NJ: Lawrence Erlbaum Associates.
Heyman, G. H., & Goodman, J. B. (submitted). Matching as
an elementary behavioral principle: a Markov analysis of
preference in concurrent choice procedures. .
Heyman, G. M., & Monaghan, M. M. (1994). Reinforcer
magnitude (sucrose concentration) and the matching law theory of
response strength. Journal of the Experimental Analysis of
Behavior, 61, 505-516.
Hoebel, B. G. (1969). Feeding and self-stimulation. Annals
of the New York Academy of Sciences, 157, 758-778.
Hursh, S. R. (1980). Economic concepts for the analysis of
behavior. Journal of the Experimental Analysis of Behavior, 34,
219-238.
Hursh, S. R., & Natelson, B. H. (1981). Electrical brain
stimulation and food reinforcement dissociated by demand
elasticity. Physiology & Behavior, 26, 509-515.
Johnson-Laird, P. N. (1988). The computer and the mind.
Cambridge, Massachusetts: Harvard University Press.
Kagel, J. K., Battalio, R. C., & Green, L. (1995). Economic
choice theory: an experimental model of animal behavior.
Cambridge: Cambridge University Press.
Kahneman, D. (1994). New challenges to the rationality
assumption. Journal of Institutional and Theoretical
Economics, 150(1), 18-36.
Kahneman, D., Fredrickson, B. L., Schreiber, C. A., &
Redelmeier, D. A. (1993). When more pain is preferred to less:
adding a better end. Psychological Science, 4(6),
401-405.
Kahneman, D., Wakker, P. P., & Sarin, R. (1997). Back to
Bentham? Explorations of experienced utility. Quarterly
Journal of Economics, 112(2), 375-405.
Lamb, R. J., Preston, K. L., Schindler, C. W., Meisch, R. A.,
Davis, F., Katz, J. L., Henningfield, J. E., & Goldberg, S.
R. (1991). The reinforcing and subjective effects of morphine in
post-addicts: a dose-response study. Journal of Pharmacology
and Experimental Therapeutics, 259(3), 1165-1173.
LeDoux, J. (1996). The emotional brain. New York: Simon
& Schuster.
Leon, M., & Gallistel, C. R. (1992). The function relating
the subjective magnitude of brain stimulation reward to
stimulation strength varies with site of stimulation. Behavioural
Brain Research, 52, 183-193.
Lepore, M., & Franklin, K. B. J. (1992). Modelling drug
kinetics with brain stimulation: Dopamine antagonist increase
self-stimulation. Pharmacol Biochem Behav, 41,
489-496.
Lilly, J. C., & Miller, A. M. (1962). Operant conditioning
of the bottlenose dolphin with electrical stimulation of the
brain. Journal of Comparative and Physiological Psychology, 55,
73-79.
Loewenstein, G. (1996). Out of control: visceral influences on
behavior. Organizational Behavior and Human Decision
Processes, 65(3), 272-292.
Macfarlane, D. B. (1954, March 12, 1954). McGill opens vast
new research field with brain "pleasure area" discovery.
The Montreal Star, pp. 1-2.
Mark, T. A., & Gallistel, C. R. (1993). Subjective reward
magnitude of medial forebrain stimulation as a function of train
duration and pulse frequency. Behavioral Neuroscience, 107(2),
389-401.
Mason, P., & Milner, P. (1986). Temporal characteristics
of electrical self-stimulation reward: fatigue rather than
adaptation. Physiology & Behavior, 36, 857-860.
Mazur, J. E. (1986). Choice between single and multiple
delayed reinforcers. Journal of the Experimental Analysis of
Behavior, 46, 67-78.
Mazur, J. E., Stellar, J. R., & Waraczynski, M. (1987).
Self-control choice with electrical stimulation of the brain. Behavioural
Processes, 15, 143-153.
McFarland, D. J., & Sibley, R. M. (1975). The behavioural
final common path. Philosophical Transactions of the Royal
Society of London B, 270, 265-293.
Meck, W. H. (1996). Neuropharmacology of timing and time
perception. Cognitive Brain Research, 3, 227-242.
Miller, G. A., Galanter, E., & Pribram, K. H. (1960). Plans
and the structure of behavior: Holt, Rinehart and Winston,
Inc.
Miller, H. L. (1976). Matching-based hedonic scaling in the
pigeon. Journal of the Experimental Analysis of Behavior, 26,
335-347.
Morgan, C. W., & Mogenson, G. J. (1966). Preference of
water-deprived rats for stimulation of the lateral hypothalamus
and water. Psychonomic Science, 6, 337-338.
Myerson, J., & Green, L. (1995). Discounting of delayed
rewards: models of individual choice. Journal of the
Experimental Analysis of Behavior, 64, 263-276.
Newsome, W. T., & Salzman, C. D. (1993). The neuronal
basis of motion perception. Ciba Foundation Symposium, 174,
217--246.
Nisbett, R. E., & Wilson, T. D. (1977). Telling more than
we can know: verbal reports on mental processes. Psychological
Review, 84(3), 231-259.
Olds, J. (1956). Pleasure centers in the brain. Scientific
American, 195, 105-116.
Olds, J. (1958). Self-stimulation of the brain. Science, 127,
315-324.
Olds, J., & Milner, P. M. (1954). Positive reinforcement
produced by electrical stimulation of septal area and other
regions of rat brain. Journal of Comparative and Physiological
Psychology, 47, 419-427.
Pfaffmann, C., Norgren, R., & Grill, H. J. (1977). Sensory
affect and motivation. Annals of the New York Academy of
Sciences., 290, 18-34.
Porter, R. W., Conrad, D. G., & Brady, J. V. (1959). Some
neural and behavioral correlates of electrical self-stimulation
of the limbic system. Journal of the Experimental Analysis of
Behavior, 2, 43-55.
Rachlin, H., Green, L., Kagel, J. H., & Battalio, R. C.
(1976). Economic demand theory and psychological studies of
choice. In G. H. Bower (Ed.), The psychology of learning and
motivation (Vol. 10, pp. 129-154). New York: Academic Press.
Rachlin, H., Kagel, J. H., & Battalio, R. C. (1980).
Substitutability in time allocation. Psychological Review, 87,
355-374.
Redelmeier, D. A., & Kahneman, D. (1996). Patients'
memories of painful medical procedures: real-time and
retrospective evaluations of two minimally invasive treatments. Pain,
66, 3-8.
Roberts, W. W. (1958). Both rewarding and punishing effects
from stimulation of posterior hypothalamus of cat with same
electrode at same intensity. Journal of Comparative and
Physiological Psychology, 51, 400-407.
Routtenberg, A., & Lindy, J. (1965). Effects of the
availability of rewarding septal and hypothalamic stimulation on
bar pressing for food under conditions of deprivation. Journal
of Comparative and Physiological Psychology, 60(2),
158-161.
Sax, L., & Gallistel, C. R. (1990). Characteristics of
spatiotemporal integration in the priming and rewarding effects
of medial forebrain bundle stimulation. Behavioral
Neuroscience, 105, 884-900.
Schreiber, C. A., & Kahneman, D. (submitted). Determinants
of the remembered utility of aversive sounds. .
Shizgal, P. (1997). Neural basis of utility estimation. Current
Opinion in Neurobiology, 7(2), 198-208.
Shizgal, P., & Conover, K. (1996). On the neural
computation of utility. Current Directions in Psychological
Science, 5(2), 37-43.
Shizgal, P., Conover, K., & Arvanitogiannis, A. (1996).
Performance for brain stimulation reward as a function of the
rate and magnitude of reinforcement. Society for Neuroscience
Abstracts, 22(1), 686.
Shizgal, P., & Matthews, G. (1977). Electrical stimulation
of the rat diencephalon: Differential effects of interrupted
stimulation on on- and off-responding. Brain Research, 129,
319-333.
Shizgal, P., & Murray, B. (1989). Neuronal basis of
intracranial self-stimulation. In J. M. Liebman & S. J.
Cooper (Eds.), The neuropharmacological basis of reward
(pp. 106-163). Oxford: Oxford University Press.
Simmons, J. M., & Gallistel, C. R. (1994). Saturation of
subjective reward magnitude as a function of current and pulse
frequency. Behavioral Neuroscience, 108, 151-160.
Stephens, D. W., & Krebs, J. R. (1986). Foraging Theory.
Princeton, NJ: Princeton University Press.
Treue, S., & Maunsell, J. H. R. (1996). Attentional
modulation of visual motion processing in cortical areas MT and
MST. Nature, 382, 539-541.
Wasserman, E. M., Gomita, Y., & Gallistel, C. R. (1982).
Pimozide blocks reinforcement but not priming from MFB
stimulation in the rat. Pharmacology Biochemistry &
Behavior, 17, 783-787.
Watanabe, M. (1996). Reward expectancy in primate prefrontal
neurons. Nature, 382, 629-632.
Williams, B. A. (1988). Reinforcement, choice, and response
strength. In R. C. Atkinson, R. J. Herrnstein, G. Lindzey, &
R. D. Luce (Eds.), Stevens' handbook of experimental
psychology: Learning and cognition (2nd ed., Vol. 2, pp.
167-244). New York: Wiley.
Wise, R. A. (1996). Addictive drugs and brain stimulation
reward. Annual Review of Neuroscience, 19, 319-340.
Yeomans, J. S. (1988). Mechanisms of brain-stimulation reward.
In A. E. Epstein & A. R. Morrison (Eds.), Progress in
Psychobiology and Physiological Psychology (Vol. 13, pp.
227-265). New York: Academic Press.
Yeomans, J. S. (1990). Principles of Brain Stimulation.
New York: Oxford University Press.
Young, P. T. (1967). Palatability: the hedonic response to
foodstuffs. In C. o. d. e. CF, W. Heidel, J. R. Brobeck, & R.
K. Crane (Eds.), Handbook of physiology. (Vol. 1, pp.
353-366). Washington,D.C.: American Physiological Society.
Zajonc, R. B. (1980). Feeling and thinking: preferences need
no inference. American Psychologist, 35(2),
151-175.







