 |
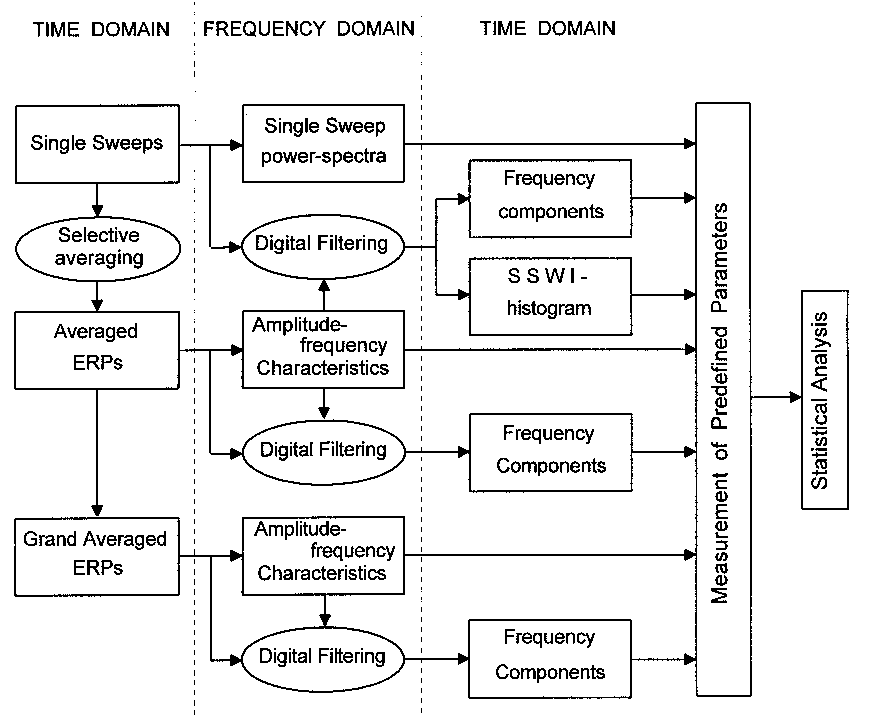
| Fig. 1. Schematic presentation of the methods and procedures used to perform time and frequency domain analysis at levels of single sweeps, averaged and grand averaged ERPs, and their relationship. |
| Children | Adults | |||||
| 6-7 years | 7-8 years | 8-9 years | 9-10 years | 10-11 years | 20-30 years | |
| Age (years) | 6.50 | 7.60 | 8.50 | 9.30 | 10.60 | 24.10 |
| SD (months) | 4.65 | 3.25 | 4.68 | 3.03 | 4.43 | 44.40 |
 |
| Fig. 1. Schematic presentation of the methods and procedures used to perform time and frequency domain analysis at levels of single sweeps, averaged and grand averaged ERPs, and their relationship. |
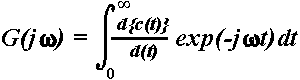
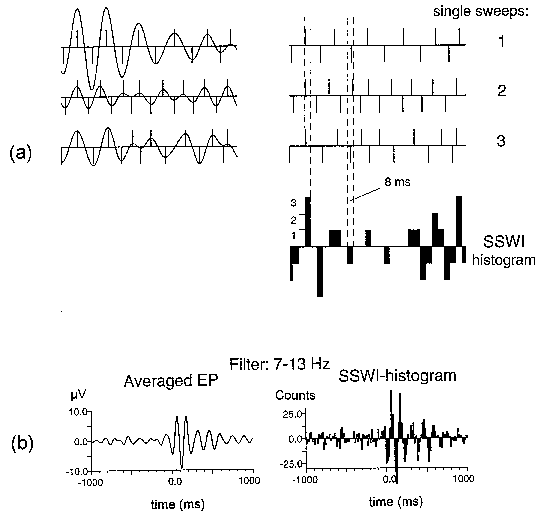 |
| Fig. 2. SSWI method. (a) Three single sweeps (1, 2, 3) filtered in the alpha range (left), the identified extrema presented by their locations with bars equal to ±1 (right), and the sums of bars in time intervals of 8 ms building the SSWI-histogram. (b) A typical result from an adult subject: averaged evoked potential filtered in the alpha range (left) and the corresponding SSWI-histogram (right). Number of single sweeps averaged and used for SSWI-histogram building is 96. Stimulus is presented at 0 ms. |
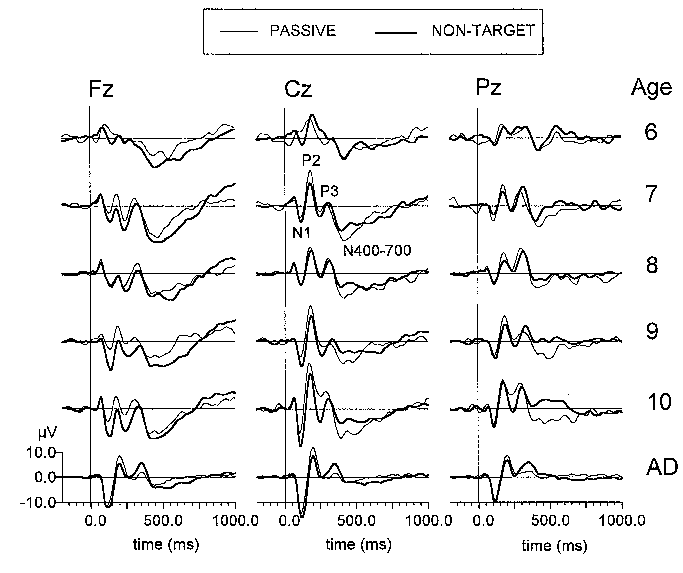 |
| Fig. 3. Grand average passive (thin line) and non-target ERPs (thick line) at three electrode locations (Fz, Cz, Pz) of six age groups (6: 6-7 year-olds, 7: 7-8 year-olds, 8: 8-9 year-olds, 9: 9-10 year-olds, 10: 10-11 year-olds, AD: adults). Each age group consists of 10 subjects. Stimulus is presented at 0 ms. |
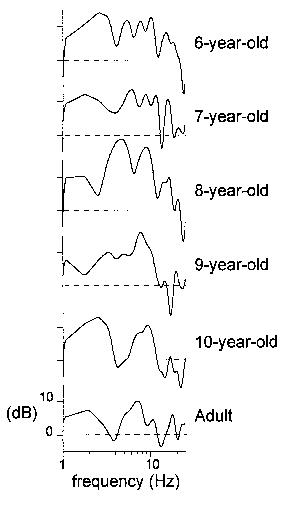 |
| Fig. 4. Amplitude-frequency characteristics for 6 representative subjects
at 6, 7, 8, 9, and 10 years of age and an adult, calculated from the passive
ERPs recorded at Cz. Along the y-axis: 20log| |
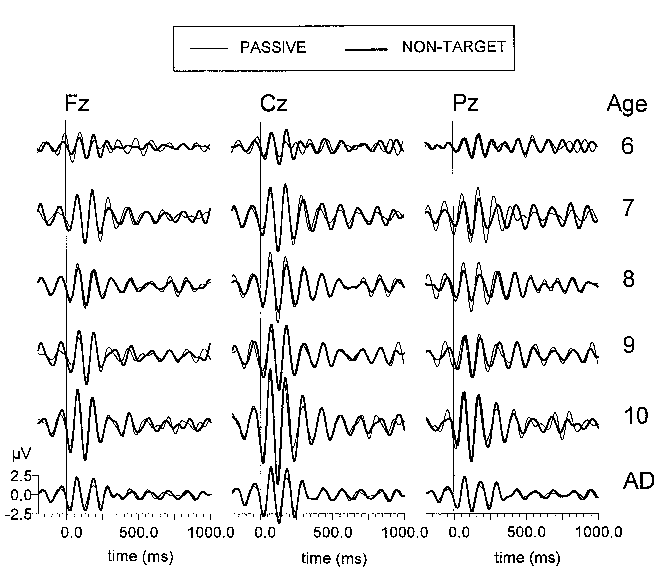 |
| Fig. 5. Grand average passive (thin line) and non-target (thick line) ERPs at three electrode locations (Fz, Cz, Pz) filtered in the alpha (8-15 Hz) range. The different age groups are designated in the same manner as in Fig. 3. Each age group consists of 10 subjects. Stimulus is presented at 0 ms. |
| Parameters | Source (df) | Passive | Non-target | ||
| F | P | F | P | ||
| Aaver | Age (5,54) | 3.5 | 0.01 | 4.1 | <0.001 |
| Electrode (2,108) | 9.2 | <0.001 | 24.7 | <0.001 | |
| A x E (10,108) | 1.8 | 0.06 | 3.1 | <0.001 | |
| Asin | Age (5,54) | 5.3 | <0.001 | 4.9 | <0.001 |
| Electrode (2,108) | 22.7 | <0.001 | 26.1 | <0.001 | |
| A x E (10,108) | 2.3 | 0.04 | 2.2 | 0.05 | |
| Np | Age (5,54) | 3.4 | 0.01 | 6.1 | <0.001 |
| Electrode (2,108) | 24.9 | <0.001 | 47.4 | <0.001 | |
| A x E (10,108) | 4.3 | <0.001 | 1.7 | 0.09 |
| Contrasts | Passive | Non-target | ||||||||||
| (df 1,54) | Aaver | Asin | Np | Aaver | Asin | Np | ||||||
| F | P | F | P | F | P | F | P | F | P | F | P | |
| 6 vs 7 |
|
|
|
|
|
|
|
|
|
|
|
|
| 6 vs 8 |
|
|
|
|
|
|
|
|
|
|
|
|
| 6 vs 9 |
|
|
|
|
|
|
|
|
|
|
|
|
| 6 vs 10 |
|
|
|
|
|
|
|
|
|
|
|
|
| 6 vs AD |
|
|
|
|
|
|
|
|
|
|
|
|
| 7 vs 8 |
|
|
|
|
|
|
|
|
|
|
|
|
| 7 vs 9 |
|
|
|
|
|
|
|
|
|
|
|
|
| 7 vs 10 |
|
|
|
|
|
|
|
|
|
|
|
|
| 7 vs AD |
|
|
|
|
|
|
|
|
|
|
|
|
| 8 vs 9 |
|
|
|
|
|
|
|
|
|
|
|
|
| 8 vs 10 |
|
|
|
|
|
|
|
|
|
|
|
|
| 8 vs AD |
|
|
|
|
|
|
|
|
|
|
|
|
| 9 vs 10 |
|
|
|
|
|
|
|
|
|
|
|
|
| 9 vs AD |
|
|
|
|
|
|
|
|
|
|
|
|
| 10vs AD |
|
|
|
|
|
|
|
|
|
|
|
|
 |
| Fig. 6. Averaged filtered ERPs (a), superimposed filtered single sweeps (b), and the corresponding SSWI-histograms (c) for six representative subjects at 6, 7, 8, 9, and 10 years of age, and an adult. Filter cut-off frequencies used are 8 and 15 Hz. All recordings are from the passive listening condition at Cz. Stimulus occurs at 0 ms. |
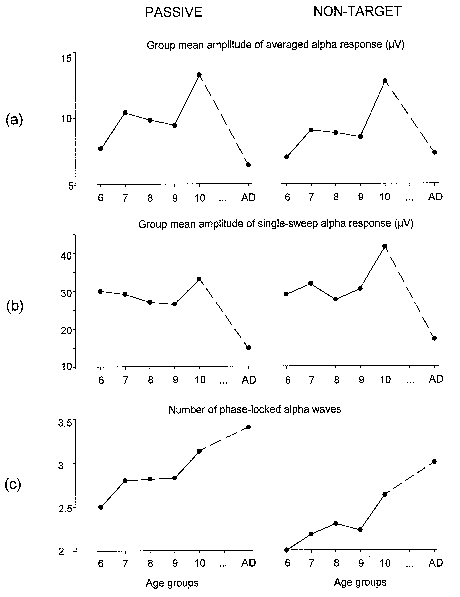 |
| Fig. 7. Group mean amplitude of alpha responses in averaged passive and non-target ERPs (a), group mean amplitude of single sweep alpha responses (b), and group means of the number of phase-locked alpha waves as calculated from normalized SSWI-histograms (c). All amplitude values are in µV, with age groups designated as in Fig. 3. Left column - passive, right column - non-target ERP. |
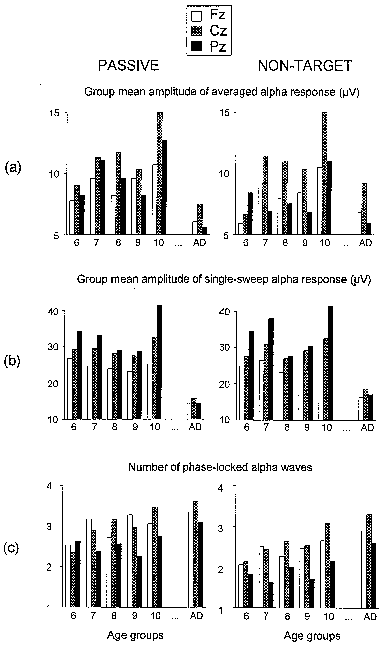 |
| Fig. 8. Group mean amplitude of averaged alpha response (a), group mean amplitude of single sweep alpha response (b), and group means of the number of phase-locked alpha waves as calculated from normalized SSWI-histograms (c) versus age at three electrode locations (Fz, Cz, Pz). All amplitude values are in µV, with age groups designated as in Fig. 3. Left column - passive, right column - non-target ERP. |
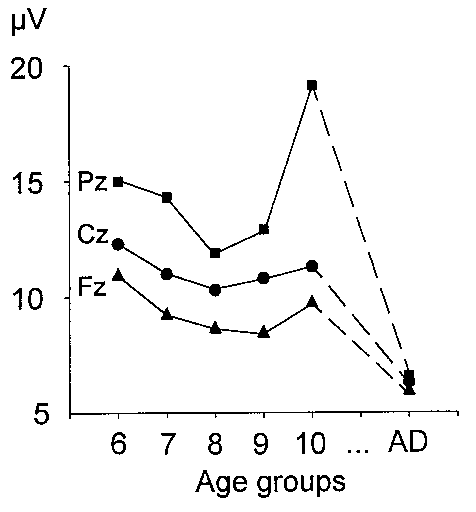 |
| Fig. 9. Mean group amplitude of the pre-stimulus alpha band activity in the passive condition at Fz, Cz and Pz. Age groups are designated as in Fig. 3. |