Original reference:
Moore, J. (1984). The evolution of reciprocal sharing. Ethol. Sociobiol. 5: 5-14.
Ethology and Sociobiology 5: 5-14 (1984)
Received Sept. 16, 1982; revised April 17, 1983.
Jim Moore
Anthropology Dept., Harvard University, Cambridge, MA 02138
[1998 Eprint: Current address: Anthropology Dept, UCSD, La Jolla CA 92093-0532]
Genetical models of the evolution of reciprocal altruism (as distinct from cooperation, mutualism, or nepotism) have difficulty explaining the initial establishment of an altruist gene in a selfish deme. Though potential mechanisms have been suggested, there is an alternative: much "altruistic" behavior may in fact be purely selfish in origin and consequently reciprocity need not be invoked to provide a selective benefit to the actor. Sharing and helping are fundamentally different behavior categories and should not be confused. Patterns of resource sharing in chimpanzees correspond to predictions made by a selfish model but not to those of a reciprocal altruism model, and many observations of human gift exchange are consistent with the selfish, but not the altruistic, model. This suggests that presumed hominid meat exchange may have been the result of competition, not altruism or even cooperation, and that evolutionary models of "altruistic" behavior should be treated with caution.
KEY WORDS: altruism, reciprocity, chimpanzee, hominid
INTRODUCTION
As emphasized by Wilson (1975, p. 3), true altruism is biologically paradoxical in that any genetic inclination towards self-sacrifice must exist in spite of that very sacrifice. Trivers addressed the problem of altruism between unrelated individuals in a landmark paper (1971). He argued that such altruism is advantageous to its practitioner if directed only toward those individuals who will reciprocate; given the right cost-benefit ratios, over time, each member of a reciprocal relationship will do better than by acting alone. As conceived by Trivers, reciprocal altruism is most likely to evolve in species with long lives, good memories, and viscous populations (those in which two individuals will meet many times and remember each other's past behavior).
There are at least two difficulties with Trivers' formulation. First, the initial altruist in a population is a lone altruist; without reciprocity the individual is at a disadvantage so the trait will not spread (Wilson, 1975, pp. 120-121). This initial disadvantage applies equally to all altruistic behavior, regardless of species, and has been addressed as a population genetics problem by several authors (Wilson 1977, Boorman and Levitt 1980, Fagen 1980, Axelrod and Hamilton 1981), all of whom have relied on arguments that some form of drift, kin or group selection is necessary to establish the altruist gene in a population. For example, failure of kin-recognition systems due to genetic or demographic changes could lead to kin-selected altruism becoming accidentally generalized to nonkin, a second set of adaptations then arising to enforce discrimination against nonkin nonreciprocators (Axelrod and Hamilton 1981). Second, among humans it is by no means clear that the reciprocal exchanges to which Trivers refers are "altruistic" in any sense of the word (Sahlins 1976). Contrary to what is often assumed, much human reciprocal gift-giving is characterized not by altruism, as we commonly use the term, but by "prestations which are in theory voluntary, disinterested and spontaneous, but are in fact obligatory and interested. The form usually taken is that of the gift generously offered; but the accompanying behavior is formal pretence and social deception, while the transaction itself is based on obligation and economic self-interest" (Mauss 1925, translation 1967; p. 1). In Trivers' model the genetical evolution of reciprocal altruism depends on mutual benefit and the approximate balance of the relationship (see below, "short term/long term"), but much of the human evidence shows either extreme self-interest or attempts by the actor to be so "altruistic" that others cannot fully reciprocate.
Both of these objections indicate that immediate self-interest plays a far larger part in reciprocal altruism than Trivers thought, and that any genetical theory of human reciprocal altruism or its component traits must account for this immediate self-interest.
DEFINITIONS
Authors discussing human altruism have tended to adopt terms from common usage without clearly defining them; words such as "altruism", "helping", "cooperation", and "sharing" all have slightly different connotations and it is important to distinguish among them. For example, although Trivers explicitly excluded cooperation (cooperative hunting) in his treatment of reciprocal altruism, Axelrod and Hamilton titled a recent paper "The evolution of cooperation", and consider cooperation, altruism and restraint during competition to be related phenomena, explicable by either kin selection or reciprocation theory (Axelrod and Hamilton 1981). In the preceding paragraphs, I have used the term "altruism" as Trivers and others have, as a single conceptual trait. For many purposes, however, it is important to make a distinction between helping and sharing.
It is not clear that sharing of resources always entails a cost to the donor, but helping another individual (e.g., in a fight, out of a river) always does. While a male baboon entering a consort fight at the instigation of another male is risking injury or death, and is certainly expending time and energy at some cost to himself (Packer 1977), a chimpanzee in possession of meat may lose nothing by giving some of it away -- as inferred from observations of chimps who abandoned or ignored kills when they had apparently had enough (Morris and Goodall 1977, Riss and Busse 1977, Nishida et al. 1979). A further difference is that although sharing can be enforced (Lee 1972, Sahlins 1965, Woodburn 1972), helping generally cannot (e.g., a baboon soliciting help in a fight would only double his problems by attacking a noncompliant potential ally, and threats from a drowning person may not be very effective).
Helping may then be defined as aid-giving behavior, always involving an immediate cost to the donor; no exchange of resources is involved, and enforcement is difficult or impossible. Sharing is the exchange of material goods; it may or may not be voluntary and need not involve an immediate cost to the donor.
Altruistic behavior is here defined as behavior that benefits an unrelated individual(s) while being detrimental (cost > benefit) to the actor in the short term. Any additional, long term benefits to the actor (which might reverse the C-B inequality) are contingent on subsequent behavior by the recipient (or others) over which the actor has no control. "Cost" and "benefit" are "defined in terms of contribution to inclusive fitness" (Trivers 1971). A reciprocal altruist, in Trivers' model, accepts short-term cost in expectation of future reciprocation and, hence, long term benefit.
Short term and Long term: "Mutual benefit" and "extreme self-interest" can be combined to produce mutualism (e.g., insect pollination of flowers), in which both parties benefit nearly simultaneously from each interaction. When the rewards are immediate for both actors, cheaters can be punished simply by terminating the interaction (in the case of simultaneous benefit) or by physical attack if the cheater tries to leave without reciprocating (when benefits are sequential with a short interval). However, as the interval between rewards increases, it becomes easier for one individual to cheat at the expense of the other; classical selection favoring selfishness should then decouple mutual benefit from self-interest. If the interval between interactions is very long - e.g., if a friend saves me from drowning and not until ten years later are the tables turned - there is little to stop the beneficiary - me - from walking away and it is difficult to biologically explain my decision to reciprocate. If, on the other hand, my friend would (somehow) benefit immediately from my salvation, there is no altruism and no paradox. Logically, there must be some length of time below which benefits are prompt and mutualism adequate to explain behavior (the "short term"; see below) and above which the potential for cheating is so great that self-interest "should" win (the "long term"), necessitating an explanation for reciprocity such as Trivers or I offer. Rigorous definitions of "short term" and "long term" need to be developed, but are not necessary for understanding or testing the model presented here.
Trivers listed five types of human behavior that he considered altruistic: (1) caring for sick, disabled or otherwise incompetent individuals, (2) sharing knowledge, (3) food sharing, (4) sharing implements, and (5) helping in times of danger. Caring for incompetent individuals includes aspects of sharing and of helping as defined above. Sharing of knowledge does not easily fit either category. However, most practical knowledge cannot be implemented without "sharing" it among all interested observers; e.g., among the !Kung San of the Kalahari there is very little active instruction of children -- they simply watch adults, and practice what they see (Draper 1976; see also Whiting and Whiting 1975, p. 180). If individuals share knowledge passively, it is unnecessary to invoke altruism of any sort to explain the phenomenon; McGrew (1977) makes a similar distinction between observational learning and instruction in chimpanzees.
In this paper, I use the specific terms helping and sharing when it is necessary to distinguish them; otherwise, both are included in the general term altruism. Cooperation does not fit the basic condition of altruism in that all the actors derive immediate benefits from the act of cooperation; classical (individual) selection is sufficient to account for the evolution of cooperative behavior (Trivers 1971). Except where specified, cooperation is not considered in this paper.
Dominance and prestige are considered to be two aspects of the same phenomenon, analogous to the distinction made by Maslow (1937) between "face-to-face" and "cultural" dominance. Both dominance and prestige are usually associated with access to resources that can affect reproductive success (RS), and individuals are expected to behave so as to maximize their status on one or both of these axes. The correlation between dominance (or prestige) and RS need not be perfect for selection to favor attributes that contribute to dominant status; likewise, dominance rankings constructed with reference to different behavioral domains (e.g., access to plant food and access to mates) need not correlate perfectly for dominance to be a meaningful concept (see, for example, Smuts 1982). No "global rank system" is implied by the finding of a positive correlation between social rank or prestige and RS among monkeys, apes, and humans of both sexes (Hausfater 1975, Riss and Goodall 1977, Gomila 1975, Hartung 1976, Chagnon 1979, Irons 1979, Dewsbury 1982).
FOOD SHARING by CHIMPANZEES and HUMANS
Chimpanzees who have meat will share it with others who beg for it (Lawick-Goodall 1968, Teleki 1973), albeit sometimes reluctantly (Nishida et al. 1979). The sharing of plant foods is less common, and occurrs primarily within families - of 457 transfers of bananas observed at Gombe, 391 (86%) were between mother and offspring, and 47 (10%) were from adult males to unrelated adult females (McGrew 1975). Food sharing among relatives clearly does not require a reciprocity theory; the discussion here will therefore be restricted to consumption of vertebrate prey (often assumed to be a key factor in human evolution - e.g., Schaller and Lowther 1969).
Chimpanzee predation and meat sharing has been discussed extensively and descriptions can be found in Lawick-Goodall (1968), Teleki (1973), Nishida et al. (1979), Kawanaka (1982), and elsewhere. The salient points for this discussion are as follows:
Sex differences - Hunting and meat consumption are primarily male activities (above authors; also Wrangham 1975, McGrew 1979, but note exceptions do occur - e.g., Morris and Goodall 1977). Teleki found that females receive meat roughly as often as do males, but they tend to get much smaller portions after most of the kill has been consumed. Familial and perhaps hormonal status play a part in females' meat consumption. Just two females, Flo and her daughter Fifi, account for over half of Teleki's observations of female participation in taking/requesting interactions, and the only female he saw receiving a "fair portion" was Gigi, who was in estrous at the time. Gigi is an infertile, possibly androgenized female (Riss and Goodall 1977), so it is not clear whether her masculinity or her estrous contributed to this success. Because meat sharing is a predominantly male activity in chimpanzees, the model presented here could be applied to "innate" sex differences in human reciprocal exchange or in the distribution of kin-directed versus nonkin "altruism", IF any such differences exist. Contemporary western culture tends to discriminate against women's participation in "public" - effectively, nonkin - affairs, and consequently demonstration of any hypothetical innate sex differences (as distinct from cultural) is probably impossible and is not attempted here. The model presented is based on genetic selfishness and an association between status and reproduction; in principle it applies equally well to females and males (see Hrdy 1981 for a review of competition among female primates).
Predation and the "control role" - During the first moments following a capture, any individual present can grab a piece - in fact, some prey are killed incidentally by dismemberment (Teleki 1973). Very shortly, though, the holder of meat becomes its possessor, and snatching pieces is more or less replaced by various forms of begging. Dominance status is not totally suspended and very low-ranking individuals do tend to lose their kills, but in general the holder assumes what Teleki terms the "control role", and otherwise dominant individuals will respect the holder's ownership. The basic picture is one of clusters of 3-4 adults around each holder of a major share, begging or trying to (unaggressively) take pieces of the prey. Fallen scraps are eaten by individuals too low-ranking to join the clusters (primarily subadults and some females) and the entire kill is consumed in from 1.5 to 9 hours (Teleki 1973).
While this behavior has been interpreted as an example of reciprocal altruism (e.g., Wilson 1975, p. 128, McGrew 1979), Wrangham (1975) has suggested that the primary motivation of the animal possessing the meat may simply be self-protection. Because chimps have a relatively open dominance hierarchy (van Lawick-Goodall 1968, Teleki 1973), the possessor of the meat (Ego) is potentially vulnerable to attack by Others who desire a share. Meat is an easily carried (and shielded) resource, so the probability of Other gaining possession of the meat with a casual threat is low, whereas the cost of a real attack is potentially high (e.g., energy loss, risk of injury). Note that if Other judges this potential cost to be less than the benefit, Ego is still at risk; furthermore, just the need to guard against a possible attack by Other is a problem for Ego: the resource may be defendable but there is no opportunity to eat it. It may therefore be in Ego's immediate interest to give up part of the kill, trading it for the opportunity to eat what is left in relative peace (for a theoretical discussion of these issues see Maynard Smith and Parker 1976).
Human sharing and exchange interactions occur in three more or less distinct classes - reciprocal exchange (the focus of Mauss' analysis and Trivers' and my models), barter or sale, and simple nonreciprocal giving. Barter needs no evolutionary "explanation", except perhaps to note that by substituting short-term for long-term balancing of interactions it acts to reduce the opportunity for selfish cheating on reciprocal exchanges. Human food sharing, surely the earliest form of nonreciprocal (as well as reciprocal) giving, has been extensively reviewed by Feinman (1979) who concludes that the vast majority of such interactions occur between related individuals and so fit the predictions of kin selection. The remaining interactions - giving to nonrelatives, with benefits to the initial altruist apparently depending on long-term reciprocation - are the focus of theories of reciprocal altruism. In contrast to intrafamilial sharing (and also to the basis of Trivers' model) such interactions are often hostile and characterized by tension and deception (Mauss 1967, Sahlins 1965, 1976). Moreover, as is apparently the case with chimpanzees, among humans the sharing of resources is socially enforced. "For a Hadza hunter to fail to share a large animal with other members of the camp in which he is living is to invite violent retribution ..." (Woodburn 1972; see also Sahlins 1965, p.166). Lee (1972) points out that homicide rates among the !Kung increase with group size, larger groups breaking apart over disputes about the distribution of food. Woodburn (1972) also states that increases in group size can cause trouble: "The more successful hunters in particular will be tempted to depart", presumably because they feel exploited by increasing numbers of slackers. Many gatherer-hunter societies have elaborate rules governing the distribution of meat; however, these rules are often only poorly self-enforced, and when opportunities to conceal meat from people outside the family arise, they are often taken (e.g., Turnbull 1961, pp. 94-108, DeVore, pers. comm.).
THE EVOLUTION OF RECIPROCAL SHARING
If Ego (an intelligent chimp) risks a severe beating whenever he or she catches something edible and tries to keep it, she or he will soon learn not to be stingy. Ego should learn quickly and will be better off learning not to be stingy rather than not to hunt. It is these qualities -- a propensity to learn the right sort of thing, and to learn it quickly -- that may have been favored by natural selection (Hamburg 1963). Because of the threat of enforcement, the propensity to learn to share with non-relatives may have evolved among chimps and humans purely through individual selection. The chimp evidence is especially significant because chimps possess all six of the characteristics Trivers considers favorable for the evolution of reciprocal altruism and yet meat sharing is the only "altruism" seen outside the contexts of kinship and sex; even the cooperative nature of their hunting has been questioned (Busse 1978, Nishida et al. 1979).
The following informal model may clarify the origins of human sharing and reciprocity. The model is based on the assumption that we evolved from chimp-like hominid ancestors, with a chimp-like social organization (cf. Reynolds 1966, McGrew 1979), who were capable of employing highly sophisticated ("political") strategies to achieve their goals (Walker Leonard 1979, Curtin 1981). Based on studies of chimpanzees, three propositions about these ancestors form the basis of the model:
Dominance (and/or prestige) is always relative: a solitary individual is in principle neither dominant nor respected. One assesses one's status relative to another by the other's behavior and physical characteristics; this assessment may or may not be conscious (Parker 1974). One's drive for dominance or prestige is then a motivation to perform those behaviors which result in submissive behavior by Other(s) toward oneself (see discussion in Hinde and Stevenson-Hinde 1976). Ego, having possession of a scarce, defendable resource, will find Other's begging rewarding; if Ego can learn, and the rewards are sufficient, she or he should learn to search/hunt for the resource and to share it with Other(s) who beg (see also Chisholm 1976). Teleki has observed that:
"Status is an important ingredient of chimpanzee social life for all age-sex classes. Young males that are in transition from adolescence to adulthood in particular strive to achieve social status. These individuals repeatedly test themselves against more mature and higher--ranking individuals. Now, if the control role is in fact functional during predation, then it is plausible to believe that an individual, and especially a low-ranking adolescent or young male who is in transition, may in the long run benefit from achieving a temporary control role. A series of such achievments could conceivably raise the social status of the individual because the cumulative effect of repeated control role activities might translate into higher social status. This adjustment may be particularly effective in establishing the relative status of an individual within his own age-sex class, which will eventually replace the older generation entirely." (1973, p. 173).
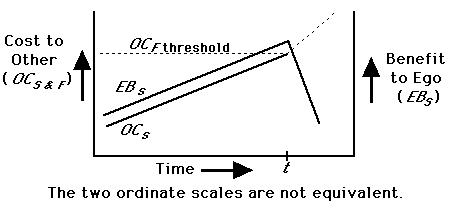
During an interaction over meat, benefits (B) and costs (C) can be partitioned into nutritional (n), status (s), and fight/risk (f) components. The nutritional value (Bn) of the meat remains constant or declines monotonically (as it is consumed) throughout the interaction; its initial value is set by its size relative to the nutritional needs of the actors. The social cost to Other (OCs) rises as Other increases the intensity of begging (until OCs > OCf) and is paralleled by Ego's Bs. If OBn > OCf, Other should then attack. At or before this point, Ego should decide whether to share or to rebuff. Dominant chimpanzee males receive portions of kills more often than those of lower rank (Teleki 1973); it seems that Ego is often (but not always) basing the decision to share on the expected reaction from Other (Fig. 1; see also Maynard Smith and Parker 1976). Ego maximizes Bs and minimizes loss in Bn by sharing just before time t (Fig. 1). Note that Ego must accurately assess both OCs and OCf in order to predict the approach of time t.

This interaction is ended; if the above inequalities were true, the resource has been shared and Ego has received a form of submission from Other, who may or may not be normally somewhat dominant to Ego. Other, if usually somewhat dominant to Ego, now "wants" to reaffirm his or her status (or, if subordinate and particularly insightful, may want to try the same trick -- this path would lead to the same results and is therefore not further considered), and has several options. Other may harrass Ego, "hoping" that Ego will behave submissively; directly challenge Ego by attacking; decide to let the incident pass; or reciprocate. The first two choices both involve the risk to Other that Ego, suddenly feeling slightly dominant, may behave as if dominant and so become dominant (Rowell 1972, p. 161). These are then dangerous options; they force an issue that is temporarily in doubt. Letting the incident pass does not force the issue, but in effect further reinforces Ego's temporary gain in status. Other individuals have seen Other beg from Ego; to maintain position in the hierarchy, Other should avoid the appearance of weakening. This line of "reasoning" is demonstrated in the phenomenon of redirected aggression, in which the loser of a fight attacks a lower-ranking bystander, demonstrating to all other individuals that his or her competitive ability and rank have not, in fact, changed (Popp and DeVore 1974, p. 61).
Other may, however, choose to reciprocate -- at some time in the near future, Other may obtain a similar valuable resource, and (assuming Other can no more monopolize it than could Ego) will want Ego to know about it and to beg for it. Ego will be only too happy to play along as long as EBn > ECs. Predicted, then, in chimpanzees, is positive selection (genetic, social, or ontogenetic) for traits associated with (aggressive) sharing and reciprocating (assertively, to re-establish status).
In humans, the hostility of potlatch-type reciprocity can be seen in this larger context. Ego, recalling Other's earlier submissive behavior, will be motivated to reciprocate and so the relationship is begun. Ego's desire to reciprocate will be matched by Other's reminding Ego of the debt, to publicly emphasize the current status relationship; the demand for repayment may originally have been implicitly or explicitly associated with the hope that the debt would remain. If the debt relationship itself becomes a major impetus towards sharing, then it follows that difficulty of repayment, not utility, will be a primary factor in selecting a gift (one suspects Christmas gifts are sometimes chosen for this reason). As Sahlins (1976) has pointed out, potlatch, kula, "big men" and other formal exchange systems are better understood in this selfish light than they are as examples of altruism.
TEST OF THE MODEL
If sharing is promoted by the expectation of future reciprocation and mutual benefit, there will be a tendency to be stingy toward old individuals who may die before reciprocating (Trivers 1971). Regarding status, Trivers makes no prediction as to choice of a reciprocal sharing partner; but it seems likely that Ego should avoid sharing with higher-ranking Others who could cheat by exploiting Ego. The present model, on the other hand, holds that if sharing is a means of avoiding attack and of gaining social status, it should be preferentially directed toward those who are most likely to attack and those who have higher status to "trade".
Examining individual success rates of eight adult male Gombe chimpanzees, Wrangham (1975) found that although there were no systematic differences among males in rates of prey capture, relative success of obtaining meat and relative age were positively correlated (Spearman r = 0.83, p<0.01). Wrangham suggests that this correlation is due to the lower reproductive value of old individuals; they risk less, in terms of potential offspring, for each unit of resource gained and are therefore more willing to attack. In fact, the two oldest males (Mike and Hugo) were involved in 90% of observed attacks over meat (N=20).
Because of social rank changes among the males during the study period, Wrangham was unable to statistically analyze the relationship between success at obtaining meat and social rank. The 3 males who held alpha rank during Wrangham's study (Mike, Figan, and Humphrey) ranked 2, 3, and 5 in success at getting meat; the fourth-ranked was Faben, brother of Figan, and the most successful male, Hugo, was old and low ranking. Similarly, Nishida (1970), Teleki (1973), and Kawanaka (1982) all conclude that high-ranking males are more likely to obtain meat than are lower ranking ones, though hierarchical relationships are greatly relaxed relative to other situations. Thus, among chimpanzee males older and/or higher-ranking individuals are in fact more likely to receive shares of meat, despite the lower probability that they will reciprocate. [NOTE ADDED 1998 eprint: I should have made clear that probability of reciprocation has not been empirically established; older/higher ranking males should have a lower probability of reciprocating, according to predictions derived from Trivers' model.]
The distribution of meat sharing, the best-known example of "reciprocal altruism" in chimpanzees, is explained by this model but not by that of Trivers.
SHARING AND RECIPROCAL ALTRUISM
Human sharing, helping and cooperating are behaviorally similar and are emotionally interconnected. It is possible that classic, selfish selection for reciprocity and aggressive sharing was responsible for establishing emotions that form the basis of the entire reciprocal altruism complex, thus bypassing the problem of the original, lone altruist. However, Packer's report of reciprocal helping among olive baboons raises the question of why baboons apparently help each other but do not share at all (Packer 1977; Harding 1973, but see Strum 1975 for a different interpretation). Clearly, Packer's finding implies (1) that aggressive sharing/reciprocity is not a necessary first step toward some forms of reciprocal altruism, and (2) sharing and helping may be quite distinct -- one need not "pull the other along" at all. I hope that future research and theorizing will clarify this issue. [1998 eprint: alternatively, baboons simply do not exhibit reciprocal altruism: Pusey & Packer 1997]
SUMMARY
The hostility surrounding reciprocal exchange of goods has intrigued researchers since the publication of "The Gift" in 1925. It is not merely the selfish demand for repayment , for often the recipient of the gift is the one insisting on making good the debt; further, some types of prestations -- potlatch, kula -- clearly are not primarily motivated by immediate economic gain. As applied to humans, Trivers' proposed model of reciprocal altruism suffers both because it does not explain the increase of the altruist genes from a very low starting frequency, and because it emphasizes the mutual benefit interpretation of reciprocal exchange -- an interpretation that is often poorly supported ethnographically. I have argued that reciprocity and aggressive sharing are directly associated with the attainment of rank in chimpanzees and humans, and may represent genetic predispositions; the mechanism of selection proposed here is distinct from that suggested by Trivers for reciprocal altruism. This model is based on a suggestion by Wrangham that sharing among chimpanzees may not be the outcome of altruism so much as a compromise solution to a standoff over resource possession. Compromise implies exchange; Teleki proposes that a part, at least, of the exchange is that of social control in return for meat, and that this temporary control may lead to a permanent change in rank. These observations readily suggest a model for the evolution of sharing: when begging is directed toward oneself it is inherently reinforcing because of the status acknowledgement implicit in it. Meat is shared, but at some social cost to the recipient; that cost can best be recouped by reciprocating at a later date. Based on analogy with chimpanzees, we can postulate that for apes moving into a hunting lifestyle, such meat/status exchanges no doubt played an important part in determining an individual's rank within the social hierarchy and hence influenced her or his RS.
This model of the origins of reciprocal sharing provides the looked-for reproductive advantage accruing to an "altruist" in a non-altruist population. Selection for traits associated with the ability to optimally take advantage of such reciprocal exchange is likely to have followed, and the critical increase of these traits in the population may have allowed true reciprocal altruism to become established; emotions evolved in one context "preadapting" individuals to take advantage of new possibilities for cooperation and helping, as well as sharing. Conscious manipulation of these emotions could then form the basis of a generalized, or societal, reciprocity ethic such as is now found in humans (Trivers 1971) and possibly odontocete cetaceans (Connor and Norris 1982). Alternatively, it is worth noting that humans and odontocetes may share another unique feature -- the ability to inflict injury or death on conspecifics without warning and with little immediate risk (humans with distance weapons such as bows, see MacDonald 1975, Lee 1979 chap. 13; odontocetes by high-intensity sonic pulses, Norris and Mohl 1981, 1983; R. Connor, pers. comm.). Little information is available yet on dolphin's use of high-intensity sound as a tool/weapon, and this point must be treated as speculative for now. If true, it would suggest that apparently altruistic helping in both groups may represent a culturally enforced extension of nepotism/sharing arising from sanctions against non-altruists rather than advantages accruing to altruists (cf. "generalized altruism", Trivers 1971). [NOTE ADDED 1998 eprint: The question of whether odontocetes use sound as a weapon remains unresolved; see Connor & Smolker 1996, Marten et al. 1988]
Note added in proof: See Western and Strum (1983) for a detailed exploration of possible relationships between gender and kin-directed versus nonkin "altruism".
ACKNOWLEDGEMENTS
I am grateful to Sarah Blaffer Hrdy, Jack Cronin, Irven DeVore, Paul Harvey, Mel Konner, David Pilbeam, Dan Rosenberg, Thom Rudegeair and Richard Wrangham for their very helpful comments on several earlier versions, and most especially to Barb Smuts for many comments, criticisms and discussions, without which this paper would likely be incomprehensible. The ideas clearly owe a great deal to the work and teaching of Robert Trivers and Richard Wrangham -- many thanks.
REFERENCES
Axelrod, R., Hamilton, W. D. The evolution of cooperation. Science 211: 1390-1396 (1981).
Boorman, S. A., Levitt, P. R. The genetics of altruism. New York: Academic Press (1980).
Busse, C. Do chimpanzees hunt cooperatively ? Am. Nat. 112:767-770 (1978).
Chagnon, N. A. Is reproductive success equal in egalitarian societies ? In Evolutionary Biology and Human Social Behavior, N. A. Chagnon and W. Irons (Eds.). North Scituate: Duxbury, 1979, pp. 374-401.
Chisolm, J. S. On the evolution of rules. In The Social Structure of Altruism, M. Chance and R. Larsen (Eds.). New York: Wiley, 1976, pp. 235-251.
Connor, R. C., Norris, K. S. Are dolphin reciprocal altruists ? Am. Nat. 119:358-374 (1982).
Curtin, R. A. Strategy and tactics in male gray langur competition. J. Hum. Evol. 10:245-253 (1981).
Dewsbury, D.A. Dominance rank, copulatory behavior, and differential reproduction. Q. Rev. Biol. 57:135-159 (1982).
Draper, P. Social and economic constraints on child life among the !Kung. In Kalahari Hunter-Gatherers, R. B. Lee and I. DeVore (Eds.). Cambridge: Harvard University Press, 1976, pp. 199-217.
Dunbar, R. I. M., Dunbar, E. P. Dominance and reproductive success among female gelada baboons. Nature 266:351-352 (1977).
Eibl-Eibesfeldt, I. Love and Hate: the Natural History of Behavior Patterns. New York: Holt, Rinehart and Winston, 1971.
Fagen, R. M. When doves conspire: evolution of non-damaging fighting tactics in a nonrandom-encounter animal conflict model. Am. Nat. 115:858-869 (1980).
Feinman, S. An evolutionary theory of food sharing. Soc. Sci. Inform. 18: 695-726 (1979).
Gomila, J. Fertility differentials and their significance for human evolution. In The Role of Natural Selection in Human Evolution, M. F. Salzano (Ed.). Amsterdam: North-Holland, 1975, pp. 155-185.
Hamburg, D. A. Emotions in the perspective of human evolution. In Expression of the Emotions in Man, P. Knapp (Ed.). New York: International Universities Press, 1963, pp. 300-317.
Harding, R. S. O. Predation by a troop of olive baboons (Papio anubis). Am. J. Phys. Anthropol. 38:587-592 (1973).
Hartung, J. On natural selection and the inheritance of wealth. Curr. Anthropol. 17:607-622 (1976).
Hausfater, G. Dominance and reproduction in baboons (Papio cynocephalus): a quantitative analysis. Contrib. Primatol. 7, Basel: S. Karger (1975).
Hinde, R. A., Stevenson-Hinde, J. Towards understanding relationships: dynamic stability. In Growing Points in Ethology, P. P. G. Bateson and R. A. Hinde (Eds.). Cambridge: Cambridge University Press, 1976, pp. 451-479.
Hrdy, S. B. The Woman That Never Evolved. Cambridge: Harvard University Press (1981).
Irons, W. Cultural and biological success. In Evolutionary Biology and Human Social Behavior, N. A. Chagnon and W. Irons (Eds.). North Scituate: Duxbury Press, 1979, pp. 257-272.
Kawanaka, K. Further studies on predation by chimpanzees of the Mahale Mountains. Primates 23:364-384 (1982).
Keverne, E. B. Social subordination and its consequences for neuroendocrine status in heterosexual groups of talapoin monkeys. Antropologia Contemporanea 3:221 (1981).
Lawick-Goodall, J. van The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Anim. Behav. Monogr. 1:165-311 (1968).
Lee, R. B. Work effort, group structure and land use in contemporary hunters and gatherers. In Man, Settlement and Urbanism, P. J. Ucko, R. Tringham and G. W. Dimbleby (Eds.). London: Duckworth, 1972, pp. 177-186.
Lee, R. B. The !Kung San. Cambridge: Cambridge University Press, 1979.
MacDonald, N. The biological factor in the etiology of war: a medical view. In War: Its Causes and Correlates, M. A. Nettleship, R. Dalegivens, and A. Nettleship (Eds.). The Hague: Mouton, 1975, pp. 209-231.
Maslow, A. H. Dominance-feeling, behavior, and status. Psychol. Rev. 44: 404-429 (1937).
Mauss, M. The Gift. New York: Norton, 1967.
Maynard Smith, J., Parker, G. A. The logic of asymetric contests. Anim. Behav. 24:159-175 (1976).
McGrew, W. C. Patterns of plant food sharing by wild chimpanzees. Proc. Int. Congr. Primatol. V:304-309 (1975).
McGrew, W. C. Socialization and object manipulation of wild chimpanzees. In Primate Biosocial Development, S. Chevalier-Skolnikoff and F. E. Poirier (Eds.). New York: Garland, 1977, pp. 261-288.
McGrew, W. C. Evolutionary implications of sex differences in chimpanzee predation and tool use. In The Great Apes, D. A. Hamburg and E. R. McCown (Eds.). Menlo Park: Benjamin/Cummings, 1979, pp. 441-463.
Morris, K., Goodall, J. Competition for meat between chimpanzees and baboons of the Gombe National Park. Folia Primatol. 28:109-121 (1977).
Nishida, T., Uehara, S., Nyundo, R. Predatory behavior among wild chimpanzees of the Mahale Mountains. Primates 20:1-20 (1979).
Norris, K. S., Mohl, B. Do odontocetes debilitate their prey acoustically ? Paper presented at the Fourth Biennial Conference on the Biology of Marine Mammals, December 1981.
Norris, K. S., Mohl, B. Can Odontocetes debilitate prey with sound? Am. Nat. 122: 85-104 (1983).
Packer, C. Reciprocal altruism in Papio anubis. Nature 265:441-443 (1977).
Parker, G. A. Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 47:223-243 (1974).
Popp, J. L., DeVore, I. Aggressive competition and social dominance theory. Unpublished manuscript (1974).
Reynolds, V. Open groups in hominid evolution. Man (Lond.) (n.s.) 1:441-452 (1966).
Riss, D. C., Busse, C. D. Fifty-day observation of a free-ranging adult male chimpanzee. Folia Primatol. 28:283-297 (1977).
Riss, D. C., Goodall, J. The recent rise to the alpha-rank in a population of free-living chimpanzees. Folia Primatol. 27:134-151 (1977).
Rowell, T. Social Behaviour of Monkeys. Baltimore: Penguin, 1972.
Sahlins, M. On the sociology of primitive exchange. In The Relevance of Models for Social Anthropology, M. Blanton (Ed.). New York: Prager, 1965, pp. 139-236.
Sahlins, M. The Use and Abuse of Biology. Ann Arbor: University of Michigan Press, 1976.
Schaffer, W. M. A note on the theory of reciprocal altruism. Am. Nat. 112: 250-253 (1978).
Silk, J. B. Patterns of food sharing among mother and infant chimpanzees at Gombe National Park, Tanzania. Folia Primatol. 29:129-253 (1978).
Silk, J. B. Kidnapping and female competition among captive bonnet macaques. Primates 21:100-110 (1980).
Strum, S. C. Primate predation: interim report on the development of a tradition in a troop of olive baboons. Science 187:755-757 (1975).
Teleki, G. The Predatory Behavior of Wild Chimpanzees. Lewisburg: Bucknell University Press, 1973.
Trivers, R. L. The evolution of reciprocal altruism. Q. Rev. Biol. 46:35-57 (1971).
Turnbull, C. M. The Forest People. New York: Simon and Schuster, 1961.
Waal, F. B. M. de, Hoekstra, J. A. Contexts and predictability of aggression in chimpanzees. Anim. Behav. 28:929-937 (1980).
Walker Leonard, J. A strategy approach to the study of primate dominance behavior. Behav. Processes 4:155-172 (1979).
Washburn, S. L., Hamburg, D. A. Aggressive behavior in old world monkeys and apes. In Primates: Studies in Adaptation and Variability, P. C. Jay (Ed.). New York: Holt, Rinehart and Winston, 1968, pp. 458-478.
Whiting, B. B., Whiting, J. W. M. Children of Six Cultures. Cambridge: Harvard University Press, 1975.
Wickler, W. Socio-sexual signals and their intra-specific imitation among primates. In Primate Ethology, D. Morris (Ed.). London: Weidenfeld and Nicolson, 1967, pp. 69-147.
Wilson, D. S. Structured demes and the evolution of group-advantageous traits. Am. Nat. 111:157-185 (1977).
Wilson, E. O. Sociobiology: the New Synthesis. Cambridge: Harvard University Press, 1975.
Woodburn, J. C. Ecology, nomadic movement and the composition of the local group among hunters and gatherers: an East African example and its implications. In Man, Settlement and Urbanism, P. J. Ucko, R. Tringham and G. W. Dimbleby (Eds.). London: Duckworth, 1972, pp. 193-206.
Wrangham, R. W. The behavioral ecology of chimpanzees in Gombe National Park, Tanzania. Ph.D. thesis, University of Cambridge, 1975.
Connor, R. C. & Smolker, R. A. (1996). 'Pop' goes the dolphin: a vocalization male bottlenose dolphins produce during consortships. Behaviour. 133: 643-662.
Marten, K., Norris, K. S., Moore, P. W. B. & Englund, K. A. (1988). Loud impulse sounds in odontocete predation and social behavior. pp. 567-579 IN Nachtigall, P. & Moore, P. W. B. (Ed.), Animal Sonar: Processes and Performance. New York: Plenum.
The following more recent references may also be of interest:
Boehm, C. (1993). Egalitarian behavior and reverse dominance hierarchy. Curr. Anthropol. 34: 227-254.
Boehm, C. (1997). Impact of the human egalitarian syndrome on Darwinian selection mechanics. Am. Nat. 150, Suppl.: S100-S121.
Connor, R. C. (1995). Impala allogrooming and the parcelling model of reciprocity. Anim. Behav. 49: 528-530.
DeNault, L. K. & McFarlane, D. A. (1995). Reciprocal altruism between male vampire bats, Desmodus rotundis. Anim. Behav. 49: 855-856.
Hemelrijk, C. K. & Lutejin, M. (1998). Philopatry, male presence and grooming reciprocation among female primates: a comparative perspective. Behav. Ecol. Sociobiol. 42: 207-215.
Mesterton-Gibbons, M. & Dugatkin, L. A. (1992). Cooperation among unrelated individuals: evolutionary factors. Q. Rev. Biol. 67: 267-281.
Peterson, N. (1993). Demand sharing: reciprocity and the pressure for generosity among foragers. Am. Anthropol. 95: 860-874.
de Waal, F. B. M. (1989). Food sharing and reciprocal obligations among chimpanzees. J. Hum. Evol. 18: 433-459.
In a recent review Pusey & Packer conclude that "animals may not be as predisposed toward reciprocity as has often been claimed. ... any claims of reciprocity seem increasingly tenuous." They do not discuss Packer's 1977 paper.
Pusey, A. E. & Packer, C. (1997). The ecology of relationships. pp. 254-283 IN Krebs, J. R. & Davies, N. B. (Ed.), Behavioural Ecology: an Evolutionary Approach (4th Edition). Oxford: Blackwell Scientific. Back to text