email: eliot@garnet.berkeley.edu
phone: (510) 642-0135
fax: (510) 642-5293
A crucial step in timing research is to isolate clock components from other sources of temporal variability. Significant progress has been made both behaviorally and neurologically. More elaborate experimental designs have helped researchers separate timing mechanisms from motoric, sensory, and mnemonic processes. Marked similarities in the temporal characteristics of the clock in perception and production tasks implicate a common timing system. Similar conclusions can be reached from studies of patient populations: Individua ls with neocerebellar damage are impaired at discriminating and reproducing short intervals. However, other patient populations, especially those with disorders affecting the basal ganglia, also exhibit deficits in timing tasks. Temporal computation may be distributed throughout the brain, but recent evidence suggests specific roles for different neural structures.
Precise timing is essential in many of our behaviors. Reaching for an object requires a specific temporal pattern of activity across the muscles of the shoulder, arm, and wrist. Similarly, predicting events, such as when a traffic light will change to red, demands an accurate estimate of the relevant durations, such as how long the light has been yellow. These examples emphasize that temporal information underlies both sensory and motoric processes; in everyday activities, intervals must be correctly perceived and produced.
For both perception and production there is, of cour se, variability in temporal accuracy. The components of a movement differ from trial to trial, and on perception tasks, we make slight misestimations of elapsed intervals. However, error can emerge from a variety of sources that do not necessarily involve timing per se, but instead results from variability in other task related processes. Movement commands may be initiated at the correct time, but delays in their implementation can occur downstream, leading to poor performance. An analogous situation exists for perception: Returning to the example of the traffic light, error in predicting the change to red can be caused by misjudging the relevant duration or from failing to rapidly detect the light's transition from green to yellow. Box 1 describes two approaches used to partition variability on temporal tasks into component sources.
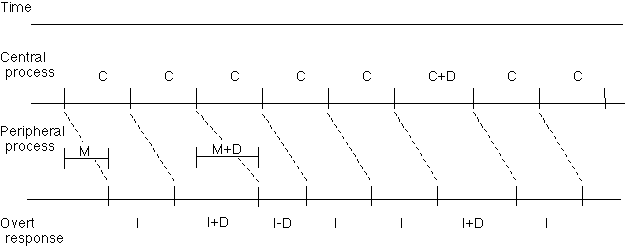
Wing and Kristofferson16 developed a simple model to analyze variability on a motor timing task. Subjects initially matched their movements to a pacing signal (e.g. 2.5 Hz), and then continued to make periodic movements after the signals terminated. Variability during the unpaced phase was hypothesized to arise from two sources: an internal clock providing an appropriately timed trigger for each movement and a motor implementation system translating this signal into a movement. Two assumptions are important for this model. First, the variability of the clock and motor implementation systems must be independent. Second, the operation of these processes must occur in an "open-loop" fashion so that no attempts are made to correct errors on previous taps. These assumptions mean that motor implementation variability causes a negative correlation between the timing of successive intervals whereas the effects of clock variability are restricted to a single interval (Figure 1). Thus, implementation variability can be identified by the covariance between successive intervals. By subtracting this estimate from the total variability, an estimate can be made of clock variability.

Figure 1. According to the Wing and Kristofferson model16, tapping variability is attributed to two processes. The central process issue tap commands about every C ms. The peripheral process requires on average M ms to implement this command. The two processes proceed independently; delays in one have no effect on the progression of the other. If implementation is delayed by D ms in the motor process (see left half of figure), the interval whose termination is defined by the tap will be lengthened, and the next interval, whose onset is defined by that tap, will be shortened. In contrast, the influence of clock variability is limited to a single interval (see right half of figure). Note that in a typical tapping task, the interval C is much longer than the motor delay M.
An alternative approach, the slope method, is based on a well-established phenomenon in the time perception literature is that variance increases linearly as a function of the square of the duration being estimated. In other words, time perception adheres to Weber's law.?a,b,c,d Ivry and Hazeltine3 applied this logic in a time production task by having subjects tap over a range of intervals. The function relating variance to target duration was very nearly linear and had a positive y-intercept. Thus, regression analyses were used to separate duration-dependent and duration-independent processes, defined by the slope and intercept terms, respectively. It was assumed that the duration-dependent variance reflected the operation of an internal clock, since other processes, such as signal detection or motor implementation, should remain constant across different durations. Close agreement is found for estimates of implementation variability calculated from the slope method or via the Wing-Kristofferson model (see also reference e).
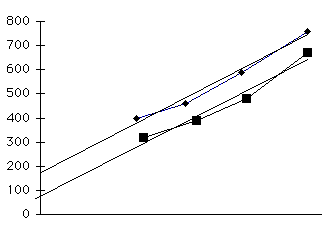
Figure 2. The variability functions for both the time production and perception tasks were best described when variance was plotted as a function of duration squared. In panel A, the target interval was presented once in both the production and perception tasks. In panel B, the target interval was presented four times in both tasks. In both experiments, the slope was comparable for the production and perception tasks. Moreover, the magnitude of the slope values was affected by the number of intervals produced or presented. This suggests that duration-dependent variability is reduced when there are multiple presentations of the target interval.
References
a. Allan, L. (1979). The perception of time. Perception & Psychophysics, 26, 340-354.
b. Bizo, L. A., & White, K. G. (1997). Timing with controlled reinforce density: Implications for models of timing. Journal of Experimental Psychology: Animal Behavioral Processes, 23, 44-55.
c. Getty, D. (1975). Discrimination of short temporal intervals: A comparison of two models. Perception and Psychophysics, 18, 1-8.
d. Gibbon, J. (1991). Origins of scalar timing. Learning and Motivation, 22, 3-38.
e. Wing, A. (1980). The long and short of timing in response sequences. In G. Stelmach and J. Requin (Eds)., Tutorials in motor behavior. New York: North-Holland.
Are perception and production subserved by the same clock? Evidence for a common timing mechanism can be observed in the tendency for movements to become automatically entrained with external stimuli. For instance, when we enter a nightclub, we immediately find ourselves moving to the beat.
Along this line, Treisman and colleagues examined how trains of evenly spaced clicks affected time perception1 and production tasks.2 In both cases, the influence of the clicks was shown to be dependent on their frequency ; particular frequencies systematically increased or decreased perceived or produced intervals. Moreover, frequencies that shortened estimates of perceived time, presumably by slowing down the clock, tended to lengthen movement times, and vice versa. This significant negative correlation between the biases observed on the perception and production tasks is consistent with the hypothesis of a common timing mechanism.
Ivry and Hazeltine3 also found performance similarities between time perception and time production with the slope method (see Box 1). Subjects produced temporal intervals by tapping and made temporal judgments across a range of durations (325 ms - 550 ms). The increase in the variance as a function of duration was comparable for the two tasks. Assuming this duration-dependent component of the variance provides an estimate of variability in an internal timing mechanism, these results implicate a common timing process in the two tasks. Studies measuring variation among individuals' perceptuomotor skills have also supported this hypothesis. Keele et al.4 observed significant correlations between performance on a repetitive tapping task and a duration discrimination task. Furthermore, acuity on the perception task was not correlated with performance on a non-temporal motor task (see also reference 5), suggesting that the correlation reflects overlap in the neural systems subserving these tasks.
Given the correlational nature of these studies, it is important to look for converging evidence for the common clock hypothesis. Neuropsychological research has provided an alternative method as well as offering insights into the neural bases of internal timing. The cerebellum has been shown to be critical for a wide variety of timing tasks. Some of the cardinal symptoms of cerebellar dysfunction, dysmetria and intentional tremor, have been attributed to a loss in coordination of the temporal pattern between antagonist muscles.6,7 Cerebellar dysarthria is most evident on sounds that require precise timing between different sets of articulators.8 Patients with cerebellar lesions also show increased variability on a repetitive tapping task9, and when the data are analyzed with the Wing-Kristofferson model (see Box 1), a dissociation is observed as a function of the locus of the pathology.10 Patients with medial cerebellar lesions suffered from marked peripheral, or motor, variability, while those with lateral cerebellar lesions were selectively impaired in central, or clock, variability.
Importantly, temporal deficits following cerebellar damage have been reported in the perceptual domain as well. Ivry and Keele9 observed that such lesions not only increased clock variability during tapping, but also impaired performance on a duration discrimination task. The patients performed comparably to control subjects on a loudness discrimination task, demonstrating that their deficits were not related to perception of the stimuli or general factors such as motivation. Cerebellar patients have also been found to perform poorly on tests of motion and velocity perception11,12, tasks which may rely on a representation of precise temporal information. Furthermore, bilateral increases in cerebellar rCBF were observed with PET when subjects judged the durations of tone intervals.13 While these imaging results are in accord with the cerebellar timing hypothesis, the activation can be attributed to a variety of sources. The timing condition was compared to one in which subjects were not required to make any sensory judgments, and thus differed in terms of the attentional, motor selection, and other cognitive demands.
The basal ganglia have also been implicated in timing functions. In addition to cerebellar activation, Jeuptner et al.13 reported regional cerebral blood flow increases in the basal ganglia (as well as temporal, prefrontal, and cingulate cortex) during their time perception task. While Ivry and Keele9 failed to find performance differences between a group of Parkinson's patients and controls on the repetitive tapping task, more recent studies have found that patients with either Huntington's or Parkinson's disease show greater variability in their inter-tap intervals than controls.14,15 Moreover, this behavioral phenomenon in PD patients is alleviated by L-dopa medication, and those with asymmetric symptoms produce more variable intervals with the more affected limb. Decomposition of the interval variability using the Wing and Kristofferson16 model (see Box 1) attributed the increased variability to both the central (clock) and peripheral (motor) sources15.
Cortical structures seem to contribute different, perhaps more integrative computations in timing tasks than those attributed to the cerebellum or the basal ganglia. Premotor or supplementary motor cortical lesions have been associated with deficits in rhythm production17. Furthermore, laterality effects have been reported on tests of temporal reproduction: For intervals ranging from 1 to 5 s, patients with precentral left hemisphere lesions tend to produce shorter intervals than control subjects, whereas patients with precentral right hemisphere lesions tend to produce longer intervals18.
Reports of timing deficits in many types of patient groups have led some researchers to conclude that temporal processing is distributed across a variety of cortical and subcortical systems (e.g. reference 15). However, the plurality of participating brain regions does not imply functional homogeneity. Theoretical models of neural clocks typically have discrete components each performing a specific operation (e.g. references 2, 19).
Pharmacological and lesion studies with rats have helped to differentiate the functions of cortical and subcortical areas implicated in temporal processing. A widely used procedure measures the frequency of animals' responses (e.g. bar presses) over time. On training trials, a reward is given to the first response made at some fixed interval after a tone or light. Learning is evaluated on trials in which no reinforcement is given, and the peak rate of responding typically occurs at the trained time of reinforcement. Drugs that target dopaminergic and cholinergic mechanisms produce a shift in the time at which the response rate peaks. Importantly, these two classes of drugs appear to change temporal processing via different mechanisms. The dopamine-related effects are initially dramatic but diminish with prolonged drug exposure. This pattern is consistent with the idea that the drug changes the speed of an internal clock, and over time the animal is able to modify its memory of the reinforcement time. In contrast, the acetylcholine-related effects, develop slowly but are more long lasting, suggesting that they affect the animal's temporal reference memory (reviewed in reference 20).
Meck20 proposed a multi-component model to account for these findings (Figure 3). A dopamine-dependent, basal ganglia system forms the pacemaker-accumulator mechanism, and an acetylcholine-dependent, frontal cortex system is associated with temporal memory and attention. Within the basal ganglia system, there is evidence for further specialization. Lesions to the substantia nigra (SN) prevent rats from learning discriminations between 20 s and 60 s intervals, whereas damage to the dorsal striatum predominantly disrupts performance on the 60 s intervals. To explain this dissociation, Meck hypothesized that the SN provides a timekeeping pulse which the dorsal striatum integrates for longer interval discriminations. Output from this integrator is compared by the frontal lobe system with reference memory. This model is also consistent with the timing behavior observed in patients with PD, who tend to underestimate durations.21 To date, the cerebellum has not been considered within the framework of this model.
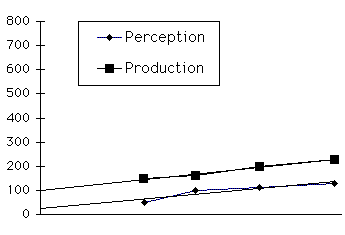
Figure 3. Meck20 proposes that the frontostriatal circuit forms a thre e component clock-counter system. The substantia nigra (SN) sends regularly paced signals throught the striatum to the pallidum, where the internal segment (GPi) acts as an accumulator. Efference from this accumulator is sent via the thalamus to the fron tal cortex, where it is compared to stored representations of task-relevant durations. This model has been proposed to account for a data from animal studies using durations of 20 s or longer. It is not clear how such a system could time the activations of different muscle groups in multijoint movements.
With some exceptions15, research examining the basal ganglia's role in timing has involved intervals spanning several seconds or more (e.g. references 20, 21, 22), while studies focusing on the cerebellum have used events of less than 1 s (e.g. references 7, 9). This confound poses the question: Are distinct neural systems recruited for these two duration ranges? Some animal research has supported this hypothesis: Cerebellar lesions in the rat led to a selective deficit on a duration discrimination task when the stimulus range was centered at 500 ms, but did not affect performance when the range was centered at 30 s. 23 Human studies also suggest a qualitative change in temporal processing for intervals longer than 1.5 - 2 s.24 For example, when attempting to tap in synchrony with a series of tones, responses are anticipatory when the inter-tone interval is less than 1800 ms; for longer intervals, the responses tend to be reactive to the tones.25
One might hypothesize that the cerebellum is critical for short intervals and other structures such as the basal ganglia and cortex take over for longer intervals. However, it remains possible that as the target interval lengthens, additional processes involved in memory and attentional functions come into play. It is important to note that the majority of the animal timing studies have not included non-temporal control tasks. For example, do dopaminergic agents distort performance when the task requires discriminations along a dimension such as stimulus intensity? Studies along this line would help develop a component analysis of temporal processing tasks, and in particular, help identify if a target neural system is specifically linked to temporal processing. This strategy has been explored in a recent study with humans.26 Cerebellar and frontal patients were tested on duration discrimination tasks and pitch discrimination tasks with the interval between the standard and comparison interval varied to either be short or long. Cerebellar patients were impaired on both duration discrimination tasks (intervals centered on 400 ms and 4 s), while frontal patients were impaired on the 4 s duration task and the pitch task when the stimuli were separated by 4 s. Such a result suggests that the frontal contribution on these tasks may not be specific to timing, but rather is manifest whenever the working memory or attentional requirements are increased.
Not only has there been a tendency to use different temporal ranges to investigate the role of the cerebellum and basal ganglia in timing, but research of these structures has emphasized different dependent variables. Timing abnormalities following cerebellar lesions are manifest as increased variability, whereas basal ganglia research has focused on changes in bias. The former effects have been viewed as evidence of a noisy timing system; the latter as a change in clock speed.
A multi-component timing mechanism can produce such deficits in a variety of ways. According to the model presented by Meck20, the dorsal striatum acts as an accumulator that stores output from other sources. A related idea builds on the hypothesis that the basal ganglia are critical for shifting cognitive set.27,28,29,30 Patients with PD take longer switching from one task to another, particularly when both use the same stimuli. In such tasks, the internal set as well as the stimuli determines the correct response. Like this form of set-shifting, timing long durations probably requires the updating of internal states in the absence of external cues. The internal states can serve as a counter, registering the accumulation of shorter subintervals. The changes in bias associated with variations in dopamine levels would then reflect the speed at which updating can occur. The pacemaker providing the short intervals could be instantiated by other structures such as the cerebellum or substantia nigra, but the critical representation of time would be related to the shifting or changing of behavioral states.31
Beginning with Braitenberg32,33, many theorists have suggested that the cerebellum's architecture is well-suited for calculating the precise temporal relationship between different inputs and between input and output patterns (see Box 2). In a recent review, Raymond et al.34 argued that the cerebellum plays a critical role in timing based on the computational overlap in two behaviors for which the structure has been shown to be critical, eyeblink conditioning (reviewed in 35) and the vestibular ocular reflex (VOR). In both cases, motor outputs (e.g. eyeblinks or eye movements) must be precisely timed to be effective.
Cerebellar Purkinje cells are the site of a tremendous convergence of information; a single Purkinje cell may receive inputs from as many as 200,000 parallel fibers. This architecture is ideal for pattern recognition.a,b These cells might learn to recognize the duration-dependent pattern of activity along the parallel fibers to signal when expected events should occur. Input to Purkinje cells could code duration in several ways. For example, granule, stellate, and basket may interact to operate much like the networks transducing temporal information to spatial information described by Buonomano and colleagues.40,41
According to this explanation, relatively slow pre- and post-synaptic mechanisms allow neurons to create local representations of temporal information. For example, Buonomano and Merzenich41 show how temporal estimates can be derived from a neural network with random variation in the time course of paired pulse facilitation and slow inhibitory postsynaptic potentials. Because of these properties, the network is in a continuously changing state after firing in response to an initial stimulus. Therefore, responses to subsequent stimulation are contingent on the length of the intervening interval.
A second hypothes is proposes that the cerebellum may house populations of interval-based timers, each coding a different duration for a particular effectorc. Just as in visual cortex specific neurons code for orientation in each region of the visual field, units in cerebellar cortex could code for a specific interval. These units could be associated with specific muscle groups, or be linked with perceptual information. When more than one effector is used, such as when an individual taps with both the left and right index fingers, output from all the units is averaged, resulting in a decrease in variability (see reference 45). This account is not mutually exclusive from the former.
Such timing mechanisms are unlikely to be adequate for intervals much greater than 1 s. Longer durations may be timed by the striatal system described above, or by the cerebellum in concert with other neural structures. For example, the cerebellum might time state changes in the frontal cortex with the basal ganglia required to effect these state changes. In this model, the cerebellum could provide the timed initiation signal while the frontal structures act as an accumulator.
Box 2 References
a. Albus, J. S. (1971). A theory of cerebellar function. Mathematical Bioscience, 10, 25-61.
b. Marr, D. A. (1969). A theory of cerebellar cortex. Journal of Physiology, London, 202, 437-470.
c. Ivry, R. (1996). The representation of temporal information in perception and motor control. Current Opinion in Neurobiology, 6, 851-857.
Why should the cerebellum, traditionally labeled a motor structure, be involved in the perception of time? In many cases, the distinction between time production and time perception is unclear. Consider, for example, when one attempts to grab a moving object, a task widely considered the purview of the cerebellum. Here, motor commands must be integrated with dynamic sensory information to properly perform the action; timing is relevant for both predicting the location of the target object and scheduling the activation of component muscle groups.
A system that learns to solve such complex problems might analyze correspondences between the two sets of events rather than independently compute the requisite temporal information for the sensory and motoric processes. Strong evidence for the cerebellum's ability to associate inputs from different sensory channels comes from experiments establishing its critical role in eyeblink conditioning 35, a learning task in which there is a need to encode the precise temporal relationships between the unconditioned stimulus and the conditioned stimulus.36,37 Perhaps this system has evolved so that it can continue to operate when there is only one source of input. For example, when the duration of a short tone must be judged, the cerebellum may compare its representation of the tone with some internal standard instead of a second stimulus or motor signal.
In motor control theory, it has been proposed that the cerebellum coordinates and fine-tunes cortical outputs by performing complex pattern recognition across a wide range of inputs.38,39 This sort of computation can be used to interpret time-varying representations of sensorimotor patterns so as to estimate elapsed durations.40,41 In other words, the cerebellum may train other brain regions to recognize and anticipate representational states by identifying activations across sets of neurons that are associated with particular intervals. This hypothesis holds some similarity to that of Courschesne and colleagues42,43, who propose that the cerebellum coordinates internal operations with anticipated sensory information. Discrete motor actions are generally less than 1 s in duration, and the hypothesized mechanisms for sustaining local neural activity are also thought to be limited to relatively short durations.41 Thus, for longer durations, such a system is unlikely to be sufficient.
A pattern recognition approach to timing could help account for some notable phenomena in the literature. Despite the hypothesis that the cerebellum is critical for timing, there is a little evidence that it behaves as a pacemaker or oscillator. 44 Moreover, the character of cerebellar timing deficits, an increase in variability without a bias to shorten or lengthen intervals, is in accord with a non-oscillatory form of computation. In addition, Helmuth and Ivry45 compared unimanual and bimanual finger tapping, and observed that the variability of each finger was reduced during the bimanual condition. When the Wing-Kristofferson analysis was applied to the data, the advantage was restricted to the clock component of the variability. The researchers interpreted this advantage as evidence that independent clocks subserved the two fingers, and the clock signals were averaged to determine movement initiation time. In the present framework, the improvement would be attributed to a gain in information to evaluate; the signal from the two fingers would have richer dynamical properties than the signal from just one.
Temporal error takes many forms, suggesting that multiple operations are performed to compute time. Human and animal studies reinforce the idea that different neural systems are essential for performance on timing tasks. The question remains how to characterize the functions of these different systems. While it is possible that the representation of temporal information is distributed across multiple systems, a more parsimonious view is that these systems make distinct contributions. The use of a range of tasks and temporal intervals is essential for understanding the relation of these neural systems to both the temporal and non-temporal component operations.
Evidence suggests that perception and production share by a common timing mechanism. How is such a system implemented?
How do ensembles of neurons provide accurate temporal information?
What are the temporal ranges of these ensembles?
How do the basal ganglia, cerebellum and frontal lobes interact to perform temporal computations?
1. Treisman, M., Faulkner, A., Naish, P. L. N., et al. (1990). The internal clock: evidence for a temporal oscillator underlying time perception with some estimates of its characteristic frequency. Perception, 19, 705-743.
2. Treisman, M., Faulkner, A., Naish, P. L. N. (1992). On the relation between time perception and the timing of motor action: Evidence for a temporal oscillator controlling the timing of movement. Quarterly Journal of Experimental Psychology, 45, 235-263.
3. Ivry, R., & Hazeltine, R. E. (1995). Perception and production of temporal intervals across a range of durations: Evidence for a common timing mechanism. Journal of Experimental Psychology: Human Perception and Performance, 21, 3-18.
4. Keele, S., Pokorny, R., Corcos, D., et al. (1985). Do perception and motor production share a common timing mechanism? Acta Psychologia, 60, 173-193.
5. Keele, S., Ivry, R., & Pokorny, R. (1987). Force control and its relation to timing. Journal of Motor Behavior, 19, 96-114.
6. Hallett, M., Shahani, B., & Young, R. (1975). EMG analysis of patients with cerebellar lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 38, 1163-1169.
7. Hore, J., Wild, B., Diener, H. C. (1991). Cerebellar dysmetria at the elbow, wrist, and fingers. Journal of Neurophysiology, 65, 563-571.
8. Ivry, R., & Gopal, H. (1992). Speech perception and production in patients with cerebellar lesions. In D. E. Meyer & S. Kornblum (Eds.) Attention and Performance Volume XIV: Synergies in Experimental Psychology, Artificial Intelligence, and Cognitive Neuroscience. Cambridge: MIT Press. pp. 771-802.
9. Ivry, R., & Keele, S. (1989). Timing functions of the cerebellum. Journal of Cognitive Neuroscience, 1, 136-152.
10. Ivry, R., Keele, S., & Diener, H. (1988). Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Experimental Brain Research, 73, 167-180.
11. Ivry, R., & Diener, H. C. (1991). Impaired velocity perception in patients with lesions of the cerebellum. Journal of Cognitive Neuroscience, 3, 355-366.
12. Nawrot, M. & Rizzo, M. (1995). Motion perception deficits from midline cerebellar lesions in human. Vision Research, 35, 723-731.
13. Jeuptner, M., Rijntjes, M., Weiller, C., Faiss, J. H., Timmann, D., Mueller, S., & Diener, H. C. (1995). Localization of cerebellar timing processes using PET. Neurology, 45, 1540-1545.
14. Freeman, J. S., Cody, F. W. J., O'Boyle, D. J., et al. (in press). Abnormalities of motor timing in Huntington's disease. Parkinsonism and Related Disorders.
15. O'Boyle, D. J., Freeman, J. S., & Cody, F. W. J. (1996). The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson's disease. Brain, 119, 51-70.
16. Wing, A. & Kristofferson, A. (1973). Response delays and the timing of discrete motor responses. Perception and Psychophysics, 14, 5-12.
17. Halsband, U., & Ito, N., Tanji, J., et al. (1993). The role of premotor and the supplementary motor area in the temporal control of movement in man. Brain, 116, 243-266.
18. von Steinbuchel, N., Wittman, M., Poeppel, E. (1996). Timing in perceptual and motor tasks after disturbances of the brain. In M. A. Pastor & J. Artieda (Eds.) Time, Internal Clocks, and Movement. New York: Elsevier. pp. 281-304.
19. Gibbon, J. & Church, R. M. (1990). Representation of time. Cognition, 37, 23-54.
20. Meck, W. H. (1996). Neuropharmacology of timing and time perception. Cognitive Brain Research, 3, 227-242.
21. Pastor, M. A., Artieda, J., Jahanshahi, M., et al. (1992). Time estimation and reproduction is abnormal in Parkinson's disease. Brain, 115,2 11-225.
22. Maricq, A. V. & Church, R. M. (1983). The differential effects of haloperidol and methamphetamine on time estimation in the rat. Psychopharmocology, 79, 10-15.
23. Clarke, S., Ivry, R., Grinband, J., et al. (1996). Exploring the domain of the cerebellar timing system. In M. A. Pastor & J. Artieda (Eds.) Time, Internal Clocks, and Movement. New York: Elsevier. pp. 143-164.
24. Fraisse, P. (1963). The psychology of time. New York: Harper.
25. Mates J., Muller, U., Radil, T., et al. (1994). Temporal integration in sensorimotor synchronization. Journal of Cognitive Neuroscience, 6, 332-340.
26. Ivry, R., & Mangles, J. (1997). The many manifestations of a cerebellar timing mechanism. Presented at the Fourth Annual Meeting of the Cognitive Neuroscience Society, March 23.
27. Brown, R. G., & Marsden, C. D. (1988). Internal versus external cues and the control of attention in Parkinson's disease. Brain, 111, 323-345.
28. Downes, J. J., Sharp, H. M., Costall, B. M., et al. (1993). Alternating fluency in Parkinson's disease. Brain, 116, 887-902.
29. Hayes, A., Davidson, M., Keele, S. W., et al. (in press). Toward a functional analysis of the basal ganglia. Journal of Cognitive Neuroscience.
30. Fimm, B., Bartl, G. Zimmermann, P., et al. (1994). Different mechanisms underlying set on external and internal cues in Parkinson's Disease. Brain and Cognition, 25, 287-304.
31. Killeen, P. R., & Fetterman, J. G. (1988). A behavioral theory of timing. Psychological Review, 95, 274-295.
32. Braitenberg, V. & Atwood, R. P. (1958). Morphological observations on the cerebellar cortex. Journal of Comparative Neurology, 109, 1-34.
33. Braitenberg, V. (1983). The cerebellum revisited Journal of Theoretical Neurobiology, 2, 237-241.
34. Raymond, J. L., Liseberger, S. G., & Mauk, M. D. (1996). The cerebellum: A neuronal learning machine? Science, 272, 1126-1131.
35. Kim, J. J., & Thompson, R. F. (1997). Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends in Neuroscience, 20, 177-181.
36. Ivry, R. (1993) Cerebellar involvement in the explicit representation of temporal information. In P. Tallal, A. Galaburda, R. R. Llinàs, & C. von Euler (eds.), Temporal Information Processing in the Nervous System: Special Reference to Dyslexia and Dysphasia. Annals New York Academy of Sciences (Vol. 682). pp. 214-230.
37. Perret, S., Ruiz B., & Mauk, M. (1993). Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. Journal of Neuroscience, 13, 1708-1718.
38. Houk, J. C. & Barto, A. G. (1992). Distributed sensorimotor learning. In G. E. Stelmach & J. Requin (Eds.) Tutorials in Motor Behavior II. New York: Elsevier. pp. 71-100.
39. Thatch, W. T. (1996). On the specific role of the cerebellum in motor learning and cognition: Clues from PET activation and lesion studies in man. Behavioral and Brain Sciences, 19, 501-502.
40. Buonomano, D. V., & Mauk, M. D. (1994). Neural network model of the cerebellum. Neural Computation, 6, 38-55
41. Buonomano, D. V., & Merzenich, M. M. (1995) Temporal information transformed into a spatial code by a neural network with realistic properties. Science, 267, 1028-1030.
42. Allen, G., Buxton, R. B., Wong, E. C., et al. (1997). Attention activation of the cerebellum independent of motor involvment. Science, 275, 1940-1943.
43. Courschesne, E., & Allen, G. (in press). Prediction and preparation, fundamental functions of the cerebellum. Learning and Memory.
44. Keating, J. G., & Thach, W. T. (1995). Nonclock behavior of inferior olive neurons: Interspike interval of Purkinje cell complex spike discharge in the awake behaving monkey is random. Journal of Neurophysiology, 73, 1329-1340.
45. Helmuth, L. L. & Ivry, R. B. (1996). When two hands are better than one: Reduced timing variability during bimanual movements. Journal of Experimental Psychology: Human Perception and Performance, 22, 278-293.