Fig. 2a: Bilateral movement. Bereitschaftspotentials preceding bilateral voluntary movements.
University Clinic of Neurology and Ludwig Boltzmann Institute of Functional
Brain Topography,
Währinger Gürtel 18-20, A-1090 Vienna, Austria
_____________________________________________________________________________
Abstract
Movement is the essence of life. Without movement one cannot achieve anything. Consequently, during evolution, movement has been optimized to highest levels of precision. Visible sign of the evolution of movement is that more and more brain has been assigned for motor purposes. A carnivore´s motor cortex is just a `dimple' in its brain supported by merely a minimal frontal cortex. In the primate, the dimple evolved into a motor strip and shifted from an anterior to a central position, in order to accommodate more and more frontal brain. Homo sapiens neanderthalensis with his sloping forehead still had less frontal cortex than Homo sapiens sapiens. In the latter, about half the cortex is pre-rolandic, assigned to motor purposes in the broader sense, i.e. including areas that supply to motor such as premotor, prefrontal, etc. areas. These frontal supplier areas to motor are involved in functions such as motivation, preparation, volition, will, planning, foresight, active anticipation, precaution, intent to act, purposefulness, goal-directedness, etc. i.e. all these functions are frontal in location, and the function of the frontal lobes seems to be almost entirely behavour-related including - in man - all the future-related behavioural aspects. A special role may be assigned to the frontomesial cortex including the supplementary motor area (SMA) and the cingulate motor area (CMA). The brain apparatus supporting motor is among the most recent and highly organized cerebral structures (neoneocortex), and the corticalization of movement developed in a saltatory way in primate phylogenesis. There are many movements man can perform, but basically there are only two primordial categories of movements: Self-initiated voluntary movements = actions and responsive (externally triggered) movements = re-actions. The first category is preceded by a Bereitschaftspotential, the latter occurs between S1 and S2 of a re-action paradigm (Walter et al. 1964). Regarding the BP, recent evidence using multichannel (64) DC-EEG (time constant [tau] = [infinity]) computer-assisted amplification proved that the earliest activity occuring is over the SMA/CMA. Only later comes the primary motor area (MI) into play. This was also shown using current source density techniques (laplacians with spline interpolation), multichannel (143) MEG and FMRI.
_____________________________________________________________________________
Introduction
While the expectancy wave (CNV, Walter et al. 1964) occurs in conjunction with a reaction time experiment, our volitional self-initiated acts are preceded by the Bereitschaftspotential (BP) or readiness potential (Kornhuber and Deecke 1964, 1965). Thus, the CNV paradigm is associated with re-actions, while the BP paradigm is associated with actions. Like the CNV, the BP has an early component and a late component. The early component is termed BP1 (see Fig. 1) and lasts from the very beginning of the BP (1-2 sec or more prior to movement onset depending on the complexity of the task) to app. ½ sec prior to movement onset. The late component is termed BP2 (see Fig. 1) and lasts from ½ sec before to 0 (sec 0 in all Figures is the onset of movement in the EMG). BP1 is symmetrical even for unilateral movement, while BP2 is larger over the contralateral hemisphere.
DC-EEG
Electroencephalography originally was the only method fast enough to record movement-related cortical events. Attempts to record movement-related potentials date back to as early as 1951, when Bates probed into the pre-movement period using a cathode ray oscilloscope on a photographic plate superimposing several movement-related epochs. He did not find any pre-movement potential, for the BP escaped him due to the short time constant ([tau]) necessary for the superposition method. Kornhuber and Deecke (1964, 1965) were lucky enough to select a [tau] of 1.2 sec in order to enable discovery of the BP. Certainly for modern standards this still is too short - attenuating BP amplitude by more than 1/3 - and subsequently we used longer and longer time constants ([tau] = 3, 5, 10, 20, etc.) until since 1988 we have been employing only recordings with [tau] = [infinity], i.e. true DC-EEG (64-channel computer-assisted DC-EEG amplifier, Lindinger et al. 1991).
Fig. 1: Early and late BP. Movement-related potentials
in different recording locations of a subject performing flexions of the
right index finger (N=1100 self-initiated movements). Bereitschaftspotential
(BP) precentro-parietally negative; frontally positive. BP1 (early component
of the BP) about 1½ to ½ sec prior to movement onset. BP2
(late component of the BP) ½ sec through movement onset (sec 0,
vertical line). Pre-motion positivity (PMP, from culmination point 90 to
80 msec prior to movement onset in all centro-parietal leads, type C-subject).
Motor potential (MP) in bipolar recording L/R precentral 60 to 50 msec
prior to movement onset. Reafferent potentials (RAP) after movement onset.
From Deecke 1974
Fig. 1 gives an example of the BP preceding voluntary right index finger flexions. We see the slow negativity of the BP starting already 1½ sec prior to the onset of movement (0, vertical line). There are two BP components. As indicated at the top of Fig. 1, we see an early, more shallow negative slope, BP1, and a late, steeper negative slope, BP2. Barrett et al. (1986) reported on three components of the BP, introducing an 'intermediate component' between BP1 and BP2. However, we never found an 'intermediate component' and believe that the pre-voluntary movement period is best described postulating two principal BP components corresponding to two principal generators. It is our hypothesis supported by many pieces of experimental evidence that the BP1 principal generator is the mesial prefrontal cortex including the supplementary motor area (SMA) and probably also the cingulate motor area (CMA, see below), while the BP2 principal generator is the primary motor cortex (MI, area 4, precentral gyrus, 'motor strip').
The BP as such has an early start of 1 to 2 sec prior to movement onset, for complex movements even up to 3 sec. For simple movements as in Fig. 1, the BP is positive in frontal leads, negative in central and parietal leads as well as at the vertex. According to theory of EEG, negativity can be related to activity of the cortical areas under study, while positivity is related to inactivity (Creutzfeldt 1983). The BP paradigm (voluntary movement paradigm) investigates internally produced movement, i.e. performed out of our free will (Deecke et al. 1969, 1976, Deecke 1987, 1990). For these volitional movements one can envisage an 'act of will' necessary to self-initiate the movement (Kornhuber et al. 1989). This certainly is a 'frontal act', and the decision of 'when to move' is thought to be elaborated by the mesial fronto-central cortex. This contains the SMA. However, there is evidence that not only SMA but also adjacent parts of the cingulate gyrus are activated prior to the onset of movement ('cingulate motor area', CMA, Shima et al. 1991).
This is the situation with unilateral voluntary movements, but how are bilateral movements organized? Experiments investigating unilateral versus bilateral movements can tell us something about the temporal order of the moments when activation starts in the two principal generators that produce the BP, SMA/CMA as the principal generator for BP1 and MI as the principal generator for BP2. When does activation start? There are three possibilities: Do both generators start activation at the same time, as the Cleveland group believes (Ikeda et al. 1993; Marsden et al. 1996), or is MI earlier than SMA, which nobody believes or is SMA activation earlier than MI activation, which we believe (Deecke 1987, Deecke and Lang 1996). Our hypothesis that SMA is upstream in the final motor cascade when it comes to channeling motivation, intention or the act of will into motor execution is demonstrated in Fig. 2
Fig. 2a: Bilateral movement. Bereitschaftspotentials
preceding bilateral voluntary movements.
A: Cerebral potentials preceding bilateral simultaneous simple monophasic index finger flexions. Monophasic means as in all our experiments that the subject performed self-initiated brisk voluntary flexions of the two index fingers that remained in flexed position returning to indifferent position only during ITI (inter trial interval). Bereitschaftspotential (BP) earliest and largest at vertex. Note that at the fronto-central midline (vertex, Cz), negativity starts already at -1.1 sec, while at the primary motor cortex (MI, C'3, C'4), BP starts significantly later: at -0.7 sec (movement onset as usual at 0 sec, vertical line).128 artifact-freee trials. Monopolar recordings against linked mastoids. C'3 or C'4 1cm anterior of C3 or C4. Detail of Fig. 1 from Kristeva and Deecke 1980
Fig 2b
B: Cerebral potentials preceding bilateral simultaneous
voluntary self-initiated index finger extensions with different loads.
Note similar finding as in A: Early BP onset over the supplementary motor
area (SMA, fronto-central midline, FCz and Cz) at -1.8 sec; significantly
later BP onset over primary motor cortex (MI, C3 and C4) at -0.8 sec. The
generally earlier BP onset times are due to the fact that the loaded movements
are complex movements as opposed to the simple movements of A. From experiments
of Cui, Huter, Lindinger, Lang and Deecke (1997)
In A, the BP preceding simple monophasic bilateral index finger flexions is shown. At the fronto-central midline (vertex, Cz), negativity starts already at -1.1 sec, while at the primary motor cortex (MI, C'3, C'4), the BP starts significantly later, at -0.7 sec. In B a recent confirmation of our early observation can be seen: Experiments involving bilateral simultaneous voluntary self-initiated index finger extensions with different loads were carried out by Cui et al. (in preparation). We see an early BP onset over the supplementary motor area (SMA, fronto-central midline, FCz and Cz) at -1.8 sec, and a significantly later BP onset over primary motor cortex (MI, C3 and C4) at -0.8 sec. The generally earlier BP onset times are due to the fact that the loaded movements of Fig. 2B are complex movements as opposed to the simple movements of Fig. 2A. We have shown that BP onset times increase with the level of complexity of the movement, the longest onset times having been observed with speaking (Deecke et al. 1986) or writing and drawing (Schreiber et al. 1983).
MEG
Experiments in movement using the magnetoencephalogram (MEG) started in 1982. Generally, all graphoelements of the EEG can also be found in the MEG. Thus, the equivalent of the Bereitschaftspotential BP has been recorded in the MEG and has been termed Bereitschaftsfeld (BF) or readiness field (RF, Deecke et al. 1982). The advantage of MEG over EEG is better localization. The disadvantage is that MEG cannot `see' pure radial equivalent current dipoles. The BF late component (BF2) has been localized in MI (area 4, Deecke et al. 1983). It reveals the typical somatotopic organization known from Penfield and Rasmussen (1950), i.e.for movements of different parts of the body, the equivalent current dipoles corresponding to BF2 were distributed in a homuncular pattern (Cheyne et al. 1991).
Sources of neural activity - identified using the non-invasive measurements of cerebral magnetic fields were found to confirm the somatotopic organization of primary motor cortex for movements of different parts of the body in normal human subjects. The somatotopic map produced with this technique revealed slight differences as compared to the classic homunculus obtained from studies using invasive cortical stimulation in epileptic patients (Penfield and Rasmussen 1950). The typical homuncular distribution of foot-, wrist-, digit-, face- and tongue movements indicated that the generators of BF2/RF2 are, indeed, located in area 4.
So far so good for the MI generator (BF2). MEG localization of the BF1 generator was less readily achieved. In the healthy subject, both SMA generators are active also in case of unilateral movement. Due to the anatomical localization of the SMA on the mesial surface of the hemisphere, the two SMAs face eachother and partially cancel one another, and the CMA activity could be a pure radial dipole that escapes MEG detection. Therefore, we made experiments using the BP paradigm in a patient with a right SMA lesion (Lang et al. 1991). The results are shown in Fig. 3.
Fig. 3: MEG in right SMA lesion. MEG recordings of the Bereitschaftsfeld (BF). Three different 200 msec time windows of the foreperiod in the patient. He had a cerebral infarction in the territory of the right anterior cerebral artery), and he performed brisk flexions of his right index finger in a volitional self-initiated manner (voluntary movement paradigm).
A: Early phase of the readiness field (BP1 or BF1) 1200 to 1000 msec prior to the first EMG activity related to the voluntary contraction. Field lines come out of the head (solid isofield lines) at the vertex Cz, they go into the head (stippled) at the frontal region corresponding to a position F1 (between F3 and Fz). Magnetic field lines envelop an electric dipole situated on the mesial surface of the left hemisphere (see projections of the dipole into the head coordinate system at the bottom). In the center (coronal) head sketch the dipole points to the left, i.e. negative intracellular currents point to the left, which means that negative extracellular currents point to the right. This is consistent with negative activity in superficial cortical layers of the left SMA. Note dipole in head coordinate system at the bottom.
B: Same situation a little later in the foreperiod -800 to -600 msec prior to the onset of movement, still BP1/BF1 period. SMA dipole still active and well-pronounced (very little residual variance). Note dipole remaining in same place as in A in head coordinate system at the bottom.
C: In the late phase of the readiness field BP2/BF2 between -200
and 0 msec prior to the onset of movement, field lines come now out of
the head at FC3 (solid isofield lines) and go into the head at FC3 (stippled),
corresponding to an electric dipole in the left precentral area (MI hand
field). Note completely different dipole location as in A and B in head
coordinate system at the bottom.This late dipole is less unequivocal, which
probably is due to the fact that the early SMA dipole is not completely
silent but still shows some activity. Modified from Lang, Cheyne, Kristeva,
Beisteiner, Lindinger and Deecke 1991
Unilateral SMA lesion was selected in order to overcome the cancellation
problem in MEG of the two opposing SMA dipoles in the normal subject. The
patient having only one remaining SMA (the left), performed voluntary flexions
with his right index finger. The results were quite convincing: In the
early phase of the readiness magnetic field (1200 to 800 msec prior to
the onset of movement, corresponding to BP1, Fig 3A) field lines were going
out of the head at the vertex (Cz) and were going into the head at a frontal
position between F3 and Fz. They thus envelopped an electric dipole on
the mesial surface of the left hemisphere in the left (intact) SMA. A similar
dipole was found during the period of 800 to 600 msec prior to the onset
of movement (Fig. 3B), still corresponding to the BP1-SMA system. However,
in the late phase of the readiness field (during BP2), in this case measured
between 200 and 0 msec prior to movement onset (Fig. 3C), activity had
shifted: Magnetic field lines now left the head at FC3 and entered
the head at FCz. This indicated an electrical dipole in the left area 4
hand representation (MI). This again supports our hypothesis that the SMA
leads the MI motor cortex activation prior to human voluntary movement.
We believe that any self-initiated movement is preceded by SMA activity
(cf. Deecke et al. 1976, and - largely founding on our BP results - Eccles
1982). The SMA/CMA system is obviously needed also for simple movements
in order to prepare for the voluntary, endogenous movement, however more
so for complicated movements and actions.
BP in SMA lesion and Parkinson akinesia
Thus, we can state: Activation of SMA/CMA leads activation of MI in
time. For this and other reasons we attribute the starting function
of voluntary action to the SMA. Patients with chronic unilateral lesions
of one SMA were examined using a voluntary movement paradigm involving
different kinds of bimanual motor sequences (Asenbaum et al. 1990). Clinically,
these patients presented with bradykinesia contralateral to the lesion,
deceleration of initial movement, switching from sequential performance
into simultaneity and frequent failure to initiate or inhibit a movement
on one side or the other. This is shown in Table I:
|
Table I: Motor dysrhythmia, dyschronokinesia. Disturbance of movement performance with simultaneous vs. sequential movements in patients having a unilateral SMA lesion: I. Disturbance of movement initiation (1) Slowing of the first
segment of movement............ N=7
II. Disturbance of movement rhythmicity
III. Disturbance of movement performance
|
The clinical syndrome of SMA lesion is termed motor dysrhythmia
or dyschronokinesia. BP (prior to movement) and negativity of
performance (N-P, post movement onset) were larger over the lesioned hemisphere.
This seemingly paradoxical result is due to the orientation of the dipole
of the remaining SMA on the mesial surface of the hemisphere. The bradykinesia
of these frontal patients resembled the one seen in basal ganglia disorder
(Parkinson's Disease, PD).
In Parkinson akinesia, the BPs are normal over the SMA but reduced and
delayed over MI (Deecke et al. 1977). On closer look, the early BP component,
BP1, is reduced, while a normal or even larger late BP component, BP2,
helps the patients to pulling up and at movement onset reaching same amplitudes
as the healthy controls - even overshooting after movement onset (Dick
et al. 1989; Harasko-van der Meer et al. 1996; Lang and Deecke 1998). This
is shown in Fig. 4.
Fig. 4: Bereitschaftspotential in Parkinson patients
and age-matched healthy controls. Ss (N=8) and patients (N=8) performed
in a typical monophasic `voluntary movement paradigm' (they performed brisk
extensions [left-sided graphs] or flexions [right-sided graphs] of their
right index finger against slight resistence, kept the target position
over at least 5 sec, and then, in the inter trial interval, ITI, moved
the finger back to initial position. Movement onset at vertical line (t
= 0 sec). Brain potentials were recorded at the vertex (Cz, over the SMA,
upper graphs) and at C3 (over the left MI hand area, lower graphs). Negativity
is up. Note that in both conditions, extension (left) and flexion (right),
the BP over SMA (Cz) in Parkinson patients (thick line) starts significantly
later and is significantly smaller than in the controls (thin line). After
the onset of movement, the situation is reversed: Amplitudes for Parkinson
patients are higher than they are for controls. Over the contralateral
motor cortex (C3) BP in Parkinson´s disease also starts later but
then becomes higher in amplitude than controls. Also note that extension
creates higher amplitudes than does flexion in both Parkinson patients
and normals. For the latter this has previously been reported (Deecke et
al. 1980). At the bottom, the trajectories of the movement are given: Movements
of the Parkinson patients are slower (upper graphs) as compared to the
healthy controls (lower graphs) for both extension (left) and flexion (right).
The findings of this Figure are first direct evidence that in patients
with Parkinson´s disease thereexists reduced cortical excitability
in the organization of voluntary movement. From Lang and Deecke 1998, data
from Harasko-van der Meer et al. 1996.
Eight Parkinson patients and eight age-matched healthy controls participated in a typical `voluntary movement paradigm'. They performed brisk monophasic extensions or flexions of the right index finger against slight resistence, kept the target position over at least 5 sec, and during the inter trial interval, ITI, moved the finger back to initial position. Brain potentials are shown in Fig. 4 at the vertex (Cz, over the SMA) and at C3 (over the left MI hand area). As is seen, in both conditions, extension as well as flexion, the BP over SMA (Cz) in Parkinson patients (thick line) starts significantly later and is significantly smaller than it is in the controls (thin line). After the onset of movement, the situation is reversed: Amplitudes for Parkinson patients are higher than they are for controls. Over the contralateral motor cortex (C3) BP in Parkinson´s disease also starts later but then gains even higher amplitudes than the controls. As can also be seen, extension creates higher amplitudes than flexion in both Parkinson patients and normals. We have previously reported on this notion using normal subjects (Deecke et al. 1980). As seen from the movement trajectories at the bottom, movements of the Parkinson patients are slower (upper graphs) as compared to the healthy controls (lower graphs) for both extension (left) and flexion (right). The findings are first direct evidence that in patients with Parkinson´s disease there is a reduced cortical excitability in the organization of voluntary movement. They also revealed that in Parkinson´s disease postsynaptic changes occur as well as presynaptic ones (dopamine depletion).
This implies dysfunction of cortical structures as a `hodological' consequence of basal ganglia dopaminergic deficiency. That is why Parkinson patients have trouble in self-initiation (action) while still being able to respond to external stimuli (re-action; Deecke et al. 1977, Deecke and Kornhuber 1978, Dick et al. 1989; Harasko-van der Meer et al. 1996; Lang and Deecke 1998; Simpson and Khuraibet 1986, 1987). These postsynaptic dysfunctions seem to be partially reversible with L-Dopa substitution (Dick et al. 1989; Feve et al. 1992). Analyses of BP topography revealed that the contribution of the SMA to the BP was reduced in Parkinson´s disease (Dick et al. 1989; Harasko-van der Meer et al. 1996; Lang and Deecke 1998; Cunnington et al. 1995; Marsden and Obeso 1994). PET studies confirmed a diminution of movement-related SMA activation (Playford et al. 1992; Jenkins et al. 1992; Rascol et al. 1992).
In primate models of Parkinson´s disease, it became now clear that the feedback circuits between cortex, basal ganglia, and thalamus are disturbed in this disease (Alexander 1994, Alexander et al. 1986, DeLong 1990). In detail it is the lack of inhibition of the inhibitory action of the Globus pallidum internum (Gpi) onto the excitatory (glutamatergic) thalamo-cortical pathway. This dopaminergic lack of inhibiting the inhibition is the intrinsic pathophysiology of the disease: Parkinson patients are akinetic because they are caught in the state of this giant Gpi-`hyperinhibition', which in the untreated severe cases downregulates their motor capacity to a complete `rien ne va plus.'
Internal pallidotomy can markedly improve akinesia (DeLong 1990, Laitinen et al. 1992). Why is it effective? Now we can understand, why: It removes this `all paralyzing' hyperinhibition from the thalamocortical output circuits. The SMA is one of the major projection areas of these thalamo-cortical circuits receiving even stronger input than MI. It has been shown that post pallidotomy, movement-related SMA activity as assessed by PET is restored in Parkinson patients (Ceballos-Baumann et al. 1994; Grafton et al. 1995). It is likely that the Parkinson-typical changes of the Bereitschaftspotential will also normalize post pallidotomy, which has to be shown yet. However, it is clear that Parkinson akinesia is the result of dysfunction of a complicated feedback circuit system, in which not only the basal ganglia but also the SMA plays an important role. The role of the CMA in this circuit has to be defined yet.
Emission CT (SPECT)
Experiments in movement have also been carried out using emission CT. Out of the two methods of this family (PET and SPECT) we so far employed SPECT. Using the compound HMPAO that shows a trapping effect in brain tissue, rCBF as the one physiological parameter and movement-related DC-potentials as the other were measured in the same 17 normal Ss. A visuomotor tracking (T) paradigm was employed (Lang et al. 1988). Trajectorial learning was required in a conflicting situation: a visual target moved on a screen and had to be tracked by moving a light stylus in the right hand on a photo detector plate in an inverted fashion (IT), e.g. movements of the target to the right side required hand movement to the left and vice versa. Compared to a normal non-inverted control task (T), IT required the development of a novel motor program and the prevention of returning to routine direct persuit. These additional demands in IT caused a relative hyperperfusion in regions including the middle frontal gyri, frontomedial cortex (containing the SMA), right basal ganglia, and left cerebellum. Correlations of rCBF values between the middle frontal gyrus and basal ganglia indicated a functional relation between these two brain structures. Trajectorial performance was accompanied by slow negative DC-potential shifts before tracking (BP) and during tracking (negativity of performance, N-P). In frontolateral and frontomedial (containing the SMA) recordings, amplitudes of DC-negativity were higher in IT than they were in T. This additional frontal negativity covaried with the success in trajectorial learning. These results substantiated, using a dual approach, our hypothesis that the frontal lobe plays an important role in trajectorial learning in providing a learning memory for novel motor skills. Cognitive skills as relevant for performing music were investigated as well, also emphasizing a role of the SMA in timing.
Other studies dealt with the investigation of motor imagery, that is we are able to see a movement or action `in our mind´s eye.' For example athletes make use of motor imagery (`mental rehearsal') before they start a trial in order to improve performance (cf. in particular diving). Their `mental representation of motor acts' is so good that they know beforehand whether a stroke for instance in golf will be a hit or a miss. We conducted a topographical study on the mental imagery of motor and other tasks (Goldenberg et al. 1989, Uhl et al. 1990). Three categories of items were to be imagined: Colours, Faces and (routes on) a Map.
The results showed that the Ss did indeed see the image `in their minds eye' and that the DC-shifts during imagery were related to creating and maintaining mental imagery. While over frontal and central areas, negative shifts did initially occur but subsequently rapidly declined, there was sustained DC-negatvity over the retrorolandic brain. Only at areas showing such sustained negativity we assumed imagery to actually occur. The large initial DC-negativity over frontal and central areas had to be explained. We believe it be reasonble to assume that these areas, in particular frontal ones, participate in the act of bringing about the imagery. While frontal areas, thus, are responsible for the generation of imagery, the imagery as such and the maintainance of it seemed to occur in retrorolandic brain regions only.
And over these areas interesting topographical differences between the three categories of imagined items (Colours, Faces and Map) were found. The level of DC-negativity was lowest for faces, medium for colours and highest for the map. How surprising that imagination of images that complex as faces generated less negativity than did imagining of colours (plain unicoloured sheets)! The explanation was - and it was confirmed by the SPECT study using 93% of the same Ss (Goldenberg et al. 1989) - that imagination of faces occured in more inferotemporal areas than colours and - in particular - the map. Negative DC-shifts in basal cortical areas were not seen by our electrodes arranged over the convexity of the skull, and, what was more, these areas could even inject a positive bias on the DC-shifts recorded via the linked ears reference electrodes. The fact that the map caused maximum overall DC-negativity also fitted this assumption because now imagery particularly involved areas of the convexity, namely the parietal lobes. The cortical DC-potential was mapped using current source density mapping algorithms (Laplacian transformation and spline interpolation; Lindinger et al. 1994). With this display, the two categories involving visual imagery (Faces and Colours) differed from the category spatial imagery (map). For the spatial map the topography was different, regional DC-negativity being distributed occipitally (but somewhat more anteriorly) and parietally (but not temporally). Since the same difference was found for visual and spatial perception, we considered our data as subserving further evidence for the postulate that imagery takes place at the same areas that elaborate the percepts.
In the HMPAO-SPECT study (Goldenberg et al. 1989), the distribution of rCBF was assessed during a resting state and during imagining either Colours or Faces - visual imagery- or a route on a Map - spatial imagery - similar as in the DC-potential experiment of Uhl et al. 1990. Imagery experiments were compared to resting state sessions during which Ss were advised to lie relaxed and 'to think of nothing'. The results showed that - as compared to the resting state - the imagination of Faces caused distinctly more regional activation than the other two imagery conditions. Statistically, imagining Faces was also the only condition that led to an increase of activity in - particularly the left - inferior occipital region which has been suggested by previous studies as being a crucial area for visual imagery (Basso et al. 1980, Goldenberg et al. 1989, Farah 1984, Goldenberg 1987, Uhl et al. 1990). Also for Colours, basal temporal and occipital regions were activated but less so than for Faces.
The imagery of routes on a Map, on the contrary, showed a tendency towards more activation in parietal visual association areas (area 7). This was another piece of evidence for a 'visual-spatial dichotomy' as suggested by Mishkin et al. 1983. It is well known that the visual modality has many submodalities such as form, disparity, colour, motion perception and others that are envisaged of being represented in different visual subsystems, some of which occupying distinct areas of the visual association cortex V1 to V7. Mishkin's distinction between object vision and spatial vision being represented in two separate visual pathways is a particularly intriguing concept which is corroborated by our two imagery studies described. Furthermore, the finding in our SPECT study (Goldenberg et al. 1989) that imagining Faces involved basal cortical areas to a greater extent than did that of Colours and the Map - the latter rather activating the parietal convexity - could well explain the above paradoxical result of our DC-potential study (Uhl et al. 1990) that the imagination of Faces yielded less overall negativity than did Colours. Since the information content of Faces is by orders of magnitude higher than the one of plain unicoloured sheets, this paradox could be explained by the greater degree of 'basality' (inferotemporal, inferooccipital) of Faces as compared to Colours. We have to be aware that there are large cortical regions which the electroencephalographer does not see. This again calls for the conduction of joint studies. From the SPECT study on imagery (Goldenberg et al. 1989) we were able to conclude as well that imagery occurs in those brain areas where perception takes place.
FMRI
The technique of functional magnetic resonance imaging allows the measurement of activation-related cerebral blood flow changes occuring with specific tasks. However, the spatial relationship between neuronal activity and functional cerebral blood flow changes is not known yet. A study was carried out in order to compare the center of neuronal activation (measured by MEG) with that of the blood flow responses (measured by FMRI, Beisteiner et al. 1995a,b). Eight Ss performed in a typical Bereitschaftspotential paradigm (self-initiated voluntary movement paradigm) of tapping with their right index finger, and the same Ss participated in both an MEG experiment and an FMRI experiment. The common center of neuronal activation (which was taken as the mean of the motor and sensory cortex MEG centers) and the center of the blood flow response were both in the contralateral rolandic hand area but about 1½ cm apart from eachother, i.e. the `neuronal signal' (MEG) and the `cerebrovascular signal' (FMRI) were not at the same location, which demanded for methodological improvements. We assume that the error is on the side of the FMRI method, and one source of localization error may be pixels with large signal amplitudes, since these pixels could stem from larger vessels that may be even remote from the center of neuronal activation (large vessel effect). Thus such dual approach, using MEG - thought to be very close to the `neuronal signal' - in addition to the `cerebrovascular signal' (FMRI) in a simple finger tapping task, should improve FMRI brain mapping results.
It was in favour of the large vessel hypothesis that results, indeed, showed a deterioration of FMRI localization quality with increasing signal amplitudes indicating increased contribution of the large vessel effect. Thus, for FMRI evaluation it is recommended to exclude pixels with large signal amplitudes in order to reduce the large vessel effect and to get closer to the neuronal signal which is the important parameter in Functional Topography of the Brain.
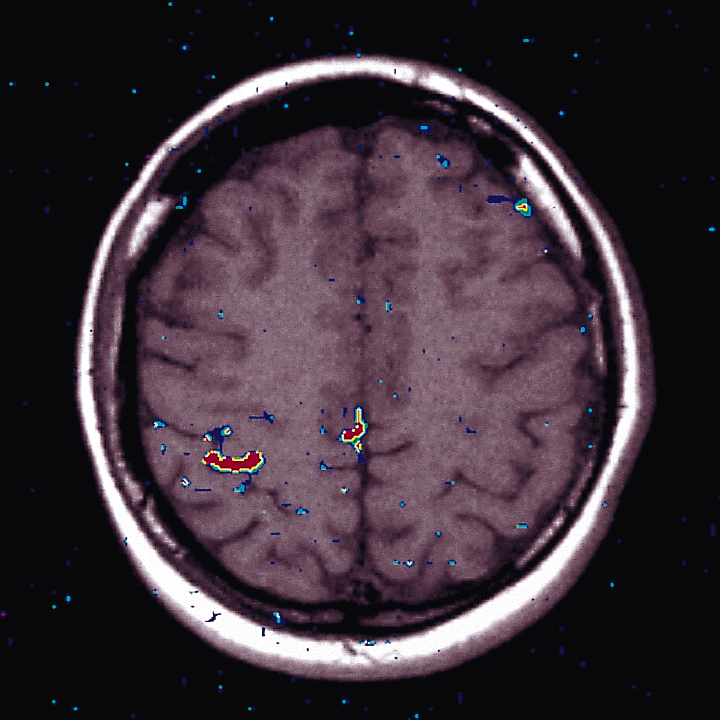
Fig. 5: Functional Magnetic Resonance Imaging. SMA participation in the Finger Tapping Task (right hand). Axial section of MRI of a subject (C.T.). Right is left. `Multicenter Study Heidelberg, Copenhagen, Vienna.' Data processing was done in Vienna. In deeper sections, both MI activity (lateral) and SMA activity (mesial) could be visualized in the same section. From Beisteiner, Erdler, Teichtmeister, Edward, Kaindl, Golaszewski, Moser and Deecke unpublished.
Recently experiments were carried out in the frame of a `Multicenter Study Heidelberg, Copenhagen, Vienna.' Data processing was done in Vienna. Fig. 5 gives the results: In addition to the rolandic (`homuncular') motor area, FMRI revealed the participation of the supplementary motor area (SMA) in the finger tapping task of the right hand. We see an axial section of the FMRI of a subject (C.T.). Right is left. In deeper sections, the two movement-related active areas MI (lateral) and SMA (mesial) can be visualized in the same section, i.e. we have another technique with which evidence could be obtained that the supplementary motor area is activated in conjunction with a voluntary movement.
Current source density
Finally we come back to the EEG. Perhaps one can say that after having been challenged by the new technique of magnetoencephalography, the electroencephalographers `stroke back.' Using special mathematics, they improved their EEG considerably with respect to spatial resolution (localisatory power and -reliability).
What is current source density? Current source density analysis is a method that allows evaluation of the topography of current sources and sinks on the scalp. Current source density is proportional to the sum of partial second derivatives of the potential field (Mackay 1984). Current source density values are proportional to the current entering and exiting the scalp. Current source density-maps are more sensitive to high spatial frequency local cortical potentials than it is to potentials of low spatial frequency due to volume conduction from distant sources (Gevins et al. 1994). Current source density analysis is independent of the location of the reference electrode used for recording. It reduces global effects caused by distant cortical sources (e.g. influences from single bad electrodes, eye movements, etc.), and enhances local surface activity by serving as a spatial filter. Furthermore, current source density analysis minimizes smearing effects as caused by the tissue transmission distortion (Nunez et al. 1994; Perrin et al. 1987). Therefore, current source density analysis arrives at an absolute and quantitative description of the field distribution, showing more precise localization of the electrical activities than the raw potential distribution (Nagamine et al. 1992). It can provide an estimation of local current density flowing perpendicularly through the skull. The current source density method is much more sensitive to local sources (both tangential and radial ones). The most significant contributions to current source density are believed to be from cortical sources (Nunez et al. 1994).
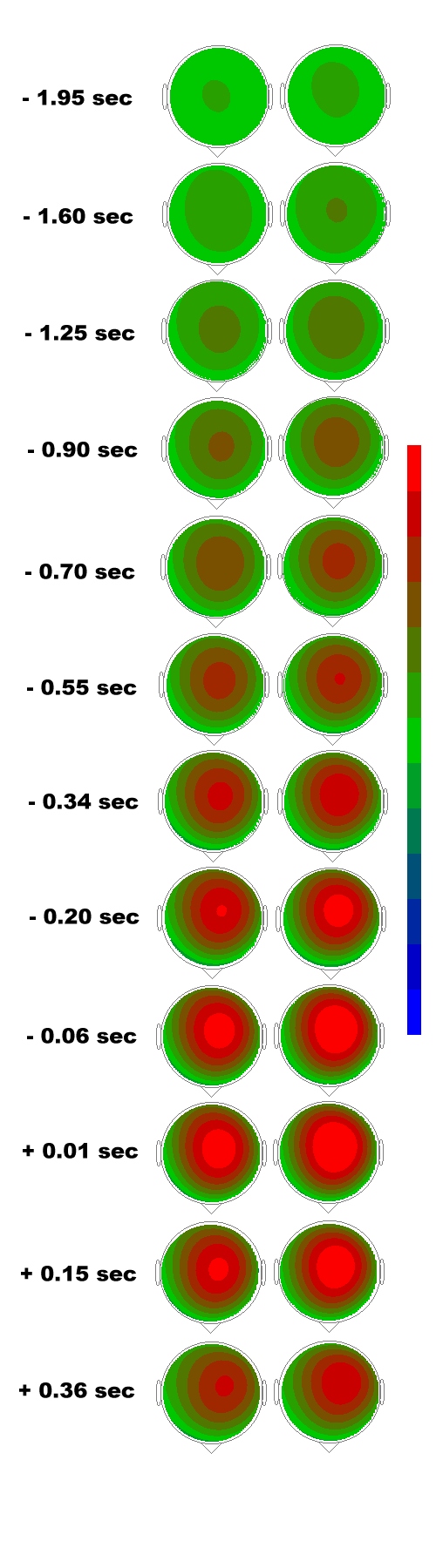
Fig. 6: Current source density. CSD maps of the
BP from the grand averages of two force loaded movement tasks, left columns,
low load, 100 pond; right columns, high load, 200 p. Maps were calculated
at time points as posted on the left. Negative times are before EMG onset,
positive times after EMG onset. The negative scale´s maximum (highest
current sink density) is represented by orange (-43 µA/mm2)
and the positive scale´s maximum by dark blue (highest current source
density). Note that with both tasks, BP starts at the fronto-central midline
(SMA/CMA)! Its early course (BP1) is bilaterally symmetrical. From -0.55
sec on, current sink (negativity) starts shifting towards the left, contralateral,
hemisphere introducing the late BP component, BP2.
Also for cortical DC-potentials current source density (Laplacian transformation and spline interpolation) is useful (Lindinger et al. 1994). Thus, the cortical DC-potential was topographically displayed using current source density mapping algorithms that enable surface topographies quite advanced over conventional mapping of the raw potentials. Fig. 6 was derived in such a way. It is taken from Cui et al. 1996. In this paper, we investigated the Bereitschaftspotential (BP) at 56 scalp positions when 23 healthy subjects performed brisk extensions of the right index finger.
These extensions in this case were performed against inertial loads of 100 g in one condition and against a resistence of 200 g in the other. The aim of the investigation was to clarify, whether the pre-movement neuronal activation in form of the Bereitschaftspotential was different e.g. in topography for the different inertial loads. Furthermore, we wanted to know, whether the early BP component (BP1) would be located in the fronto-central midline for finger extension with different loads, and finally we were interested to find out whether the activation of the SMA does occur prior to that of the primary motor area (MI)? The BP was recorded on the scalp using a 64 channel DC amplifier system.
The Results showed that amplitudes of the BP were significantly different in Cz (vertex) and C5/P3 (midway between 10-20 electrode positions C5 and P3). The comparison of Cz with C5/P3 showed that the BP amplitudes were higher in Cz than they were in C5/P3 for both tasks, especially the early component BP1. The comparison of the 3 motor areas (SMA, contralateral and ipsilateral MI) showed that the BP was largest in the SMA and smallest in the ipsilateral MI in both tasks. The current source density laplacians revealed that a clear current sink (= surface negativity) of the BP appeared on the scalp as early as 2.3 sec before the onset of movement in the EMG. This early BP onset is due to the fact that the loaded movements constitute complex movements as compared to simple ones that usually have later BP onsets of about 1.5 sec. The late component of the Bereitschaftspotential (BP2), starting about ½ sec prior to movement onset, which always is lateralized towards the contralateral hemisphere, accordingly showed a lateralized current sink. In earlier papers we called this the contralateral preponderance of negativity, CPN (Deecke et al. 1976; Deecke 1987, 1990). The CPN holds for hand movements, while foot movements are preceded by an ipsilateral preponderance of negativity (IPN, Boschert et al. 1983; Boschert and Deecke 1986). The location of the motor potential´s (MP) current sink was similar as that of BP2, but the areas and the densities were highest. We concluded that the more force is needed for finger extension against load, the larger the BP in motor areas. This is probably due to the higher effort necessary for preparing the voluntary movement against force. The early BP component (BP1) started in the SMA, while BP2 and the MP were laterialized in form of the CPN. The activation of the SMA occurred considerably earlier than the one of the MI.
In recent experiments, the spatial pattern of the Bereitschaftspotential has been analyzed in short time intervals (35 and/or 70 msec) starting 2.51 sec before movement onset. For each time segment a spherical model of the BP was calculated by using spline interpolation. Subsequently, the spatial distribution of the electric potential at the scalp surface was transformed into a spatial distribution of current source densities (CSD map). In 13 of 17 subjects there was a significant current sink in the scalp area located over the fronto-central mesial cortex (SMA/CMA) in the absence of a significant current sink over the lateral motor cortex. In three subjects significant current sinks were present at both sites and in another three subjects a current sink only over the lateral motor cortex was observed. It was concluded that there is a large group of subjects (13/17) in whom the early component of the BP is associated with a significant current sink over SMA and CMA. At a later time interval (0.6 to 0.5 sec before movement onset), current sinks in mesial and lateral positions were found in most subjects (10 of 17). These data were considered to be consistent with the hypothesis that - at least in the majority of subjects - the fronto-central mesial cortex is activated earlier than the primary motor cortex when preparing for a voluntary movement. It is clear then that our postulate that the SMA/CMA activity is upstream of MI activity holds for the vast majority of Ss but not for all, which might explain controversies with other groups (e.g. Ikeda et al. 1993). In new experiments we are presently attempt to clarify whether this difference is an intrinsic inter Ss difference or rather due to different strategies: purposeful conscious actions on the one hand or more automatic routine performance on the other.
ACKNOWLEDGEMENT
This work was supported by a research grant from the Human Frontier Science Program.
REFERENCES
Alexander GE, DeLong MR, Strick PL: Parallel organization of functionally aggregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9: 357-381 (1986)
Alexander G: Basal ganglia - thalamocortical cortical circuits: Their role in control of movements. J Clin Neurophysiol 11: 420-431 (1994)
Asenbaum S, Oldenkott B, Lang W, Lindinger G, Deecke L: Motor sequences in patients with unilateral SMA lesions: Movement-related potentials and motor disorders. In: Brunia CHM, Gaillard AWK, Kok A (Eds.): Psychophysiological Brain Research. Vol. I, Tilburg University Press, pp. 119-123 (1990)
Barrett G, Shibasaki H,. Neshige R: Cortical potentials preceding voluntary movement: evidence for three periods of preparation in man. Electroenceph Clin Neurophysiol 63(4): 327-339 (1986)
Basso A, Bisiach E, Luzatti C: Loss of mental imagery: a case study. Neuropsychologia 18: 435-442 (1980)
Bates JAV: Electrical activity of the cortex accompanying movements. J Physiol (Lond) 113: 240 (1951)
Beisteiner R, Gomiscek G, Erdler M, Teichtmeister C, Moser E, Deecke L: Funktionelles Magnet Resonanz Imaging (FMRI) - Ergebnisse und Kombinationsmöglichkeiten mit der Magneto- und Elektroenzaphalographie. In: W Lang, L Deecke, HC Hopf (Eds): Topographische Diagnostik des Gehirns. Springer Wien Vh Dt Ges Neurol 9: 189-192 (1995a)
Beisteiner R, Gomiscek G, Erdler M, Teichtmeister C, Moser E, Deecke L: Comparing localization of conventional functional magnetic resonance imaging and Magnetoencephalography. Eur J Neurosci 7:1221-1224 (1995b)
Boschert J, Hink RF, Deecke L: Finger movement versus toe movement-related potentials: further evidence for supplementary motor area (SMA) participation prior to voluntary action. Exp Brain Res 52(1): 73-80 (1983).
Boschert J, Deecke L: Cerebral potentials preceding voluntary toe, knee and hip movements and their vectors in human precentral gyrus. Brain Res 376(1): 175-9 (1986).
Ceballos-Baumann AO, Obeso JA, Vitek JL, DeLong MR, Bakay R, Linazasoro G, Brooks DJ: Restoration of thalamocortical activity after postero-ventral pallidotomy in Parkinson´s disease. Lancet 344: 814 (1994)
Cheyne D, Kristeva R, Deecke L: Homuncular organization of human motor cortex as indicated by neuromagnetic recordings. Neurosci Lett 122: 17-20 (1991)
Creutzfeldt OD: Cortex cerebri. Springer Berlin, Heidelberg New York, Tokyo pp 145-149 (1983)
Cui RQ, Huter D, Lang W, Lindinger G, Beisteiner R, Deecke L: Multichannel DC current source density mapping of the Bereitschaftspotential in the supplementary and primary motor area preceding differently loaded movements. Brain Topography. 9(2): 83-94 (1996).
Cunnington R, Iansek R, Bradshaw JL, Phillips JG: Movement-related potentials in Parkinson´s disease: Presence and predictability of temporal and spatial cues. Brain 118: 935-950 (1995)
Deecke L, Scheid P, Kornhuber HH: Distribution of readiness potential, pre-motion positivity, and motor potential of the human cerebral cortex preceding voluntary finger movements. Exp Brain Res 7: 158-168 (1969)
Deecke L: Die corticalen Potentiale des Menschen vor raschen willkürlichen Fingerbewegungen. Habilitationsschrift. University of Ulm Library (1974)
Deecke L, Grözinger B, Kornhuber HH: Voluntary finger movement in man: Cerebral potentials and theory. Biol Cybern 23: 99-119 (1976)
Deecke L, Englitz H-G, Kornhuber HH, Schmitt G: Cerebral potentials preceding voluntary movement in patients with bilateral or unilateral Parkinson akinesia. In: Desmedt JE. (Ed): Attention, voluntary contraction, and event-related cerebral potentials. Prog Clin Neurophysiol Vol 1, pp 151-163 (1977)
Deecke L, Kornhuber HH: An electrical sign of participation of the mesial ,,supplementary" motor cortex in human voluntary finger movement. Brain Res 159: 473-476 (1978)
Deecke L, Eisinger H, Kornhuber HH: Comparison of Bereitschaftspotential, pre-motion positivity and motor potential preceding voluntary flexion and extension movements in man. In: Kornhuber HH, Deecke L (Eds) Motivation, motor and sensory processes of the brain: Electrical potentials, behaviour and clinical use. Amsterdam, Elsevier, Prog Brain Res Vol 54: pp 171-176 (1980)
Deecke L, Weinberg H, Brickett P: Magnetic fields of the human brain accompanying voluntary movement. Bereitschaftsmagnetfeld. Exp Brain Res 48: 144-148 (1982)
Deecke L, Boschert J, Weinberg W, Brickett P: Magnetic fields of the human brain (Bereitschaftsfeld) preceding voluntary foot and toe movements. Exp Brain Res 52: 81-86 (1983)
Deecke L, Engel M, Lang W, Kornhuber HH: Bereitschaftspotential preceding speech after holding breath. Exp Brain Res 65: 219-223 (1986)
Deecke L: Bereitschaftspotential as an indicator of movement preparation in supplementary motor area and motor cortex. In: R. Porter (Chair), Motor Areas of the Cerebral Cortex. Wiley. Chichester, Ciba Found Symp 132: pp 231-250 (1987)
Deecke L: Electrophysiological correlates of movement initiation. Rev Neurol 146/10: 612-619 (1990)
Deecke L, Lang W: Generation of movement-related potentials and fields in the supplementary sensorimotor area and the primary motor area. In: Lüders HO (Ed.) Supplementary Sensorimotor Area - Advances in Neurology. Vol 70 Lippincott/Raven Publishers pp 127-146 (1996)
DeLong MR: Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281-285 (1990)
Dick JPR, Rothwell JC, Day BL, Cantello R, Buruma O, Gioux M, Benecke R, Berardelli A, Thompson PD, Marsden CD: The Bereitschaftspotential is abnormal in Parkinson´s disease. Brain 112: 233-244 (1989)
Eccles JC: The initiation of voluntary movements by the supplementary motor area. Arch Psychiat Nervenkr 231: 423-441 (1982)
Farah MJ: The neurological basis of mental imagery: a componential analysis. Cognition 18: 245-272 (1984)
Feve AP, Bathien N, Rondot P: Chronic administration of L-Dopa affects the movement-related cortical potentials of patients with Parkinson´s disease. Clin Neuropharmacol 15: 100-108 (1992)
Gevins A, Cutillo B, DuRousseau D, Le J, Leong H, Martin N, Smith ME, Bressler S, Brickett P, McLaughlin J, Barbero N, and Laxer K: Imaging the spatiotemporal dynamics of cognition with high-resolution evoked potential methods. Human Brain Mapping. 1: 101-116 (1994).
Goldenberg G: Neurologische Grundlagen bildlicher Vorstellungen, Springer Wien, New York (1987)
Goldenberg G, Podreka I, Uhl F, Steiner M, Willmes K, Deecke L: Cerebral correlates of imagining colours, faces and a map - I. SPECT of regional cerebral blood flow. Neuropsychologia 27: 1315-1328 (1989)
Grafton ST, Waters C, Sutton J, Lew MF, Coudwell W: Pallidotomy increases activity of motor association cortex in Parkinson´s disease: A positron emission tomographic study. Ann Neurol 37: 776-783 (1995)
Harasko-van der Meer C, Gerschlager W, Lalouschek W, Lindinger G, Deecke L, Lang W: Bereitschaftspotential preceding onset and termination of a movement is abnormal in Parkinson´s disease. Movement Disorders 11 Suppl 1: 84 (1996)
Ikeda A, Lüders HO, Burgess RC, Shibasaki H: Movement-related potentials associated with single and repetitive movements recorded from human supplementary motor area. Electroenceph Clin Neurophysiol 89: 269-277 (1993)
Jenkins IH, Fernandez W, Playford ED, Lees AJ, Frackowiak REJ, Passingham RE, Brooks DJ: Impaired activation of the supplementary motor area in Parkinson´s disease is reversed when akinesia is treated with apomorphine. Ann Neurol 32: 749-767 (1992)
Kornhuber HH, Deecke L: Hirnpotentialänderungen beim Menschen vor und nach Willkürbewegungen, dargestellt mit Magnetbandspeicherung und Rückwärtsanalyse. Pflügers Arch 281: 52 (1964)
Kornhuber HH, Deecke L: Hirnpotentialänderungen bei Willkürbewegungen und passiven Bewegungen des Menschen: Bereitschaftspotential und reafferente Potentiale. Pflügers Arch 284: 1-17 (1965) "Citation Classic"
Kornhuber HH, Deecke L, Lang W, Lang M, Kornhuber A: Will, volitional action, attention and cerebral potentials in man: Bereitschaftspotential, performance-related potentials, directed attention potential, EEG spectrum changes. Chapter 6 in: WA Hershberger (Ed) Volitional action, Elsevier (North Holland), Amsterdam, pp 107-168 (1989)
Kristeva R, Deecke L: Cerebral potentials preceding right and left unilateral and bilateral finger movements in sinistrals. In: HH Kornhuber, L Deecke (eds): Motivation, motor and sensory processes of the brain: Electrical potentials, behaviour and clinical use. Progr Brain Res Vol 54 Elsevier Amsterdam 748-54 (1980)
Laitinen LV, Bergenheim AT, Hariz MI: Leksell´s posteroventral pallidotomy in the treatment of Parkinson´s disease. J Neurosurg 76: 53-61 (1992)
Lang W, Lang M, Podreka I, Steiner M, Uhl F, Suess E, Müller C, Deecke L: DC-potential shifts and regional cerebral blood flow reveal frontal cortex involvement in human visuomotor learning. Exp Brain Res 71: 353-364 (1988)
Lang W, Cheyne D, Kristeva R, Beisteiner R, Lindinger G, Deecke L: Three-dimensional localization of SMA activity preceding voluntary movement. A study of electric and magnetic fields in a patient with infarction of the right supplementary motor area. Exp Brain Res 87: 688-695 (1991)
Lang W, Deecke L: Psychophysiologie der Motorik. Chapter 5 in: F Rösler (Ed) Volume 5 Ergebnisse und Anwendungen der Psychophysiologie. Enzyklopädie der Psychologie, Serie I Biologische Psychologie. Hogrefe Göttingen, pp 225-283 (1998)
Lindinger G, Svasek P, Lang W, Deecke L: PC-supported 64-channel DC-EEG amplifier. In: Adlassnig KP, Grabner G, Bengtsson S, Hansen R (Eds) Lecture notes in medical informatics 45. Springer Berlin pp 1005-1009 (1991)
Lindinger G, Baumgartner C, Burgess R, Lüders H, Deecke L: Topographic analysis of epileptic spikes using spherical splines and spline laplacian (CSD). J Neurol 241: 276-277 (1994)
Marsden CD, Deecke L, Freund H-J, Hallett M, Passingham RE, Shibasaki H, Tanji J, Wiesendanger M: The functions of the supplementary motor area: Summary of a workshop. Advances in Neurology, Vol. 70: Supplementary Sensorimotor Area, HO Lüders (Ed) pp 477-487 (1996)
Marsden CD, Obeso JA: The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson´s disease. Brain 117: 877-897 (1994)
Mackay MD: Source density analysis of scalp potentials during evaluated action I. coronal distribution. Exp Brain Res 54: 73-85 (1984).
Mishkin M, Ungerleider LG, Macko KA: Object vision and spatial vision: the two cortical pathways. Trends Neurosci 6: 414-417 (1983)
Nagamine T, Kkaji R, Suwazono S, Hamano T, Shibasaki H, Kimura J: Current source density mapping of somatosensory evoked responses following median and tibial nerve stimulation. Electroencephalogr Clin Neurophysiol 84: 248-256 (1992).
Nunez PL, Silberstein RB, Cadusch PJ, Wijesinghe RS, Westdorp AF, Srinivasan R: A theoretical and experimental study of high resolution EEG based on surface Laplacians and cortical imaging. Electroencephalogr Clin Neurophysiol 90: 40-57 (1994).
Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RSJ, Brooks DJ: Impaired mesial frontal and putamen activation in Parkinson´s disease: a positron emission tomography study. Ann Neurol 32: 151-161 (1992)
Penfield W, Rasmussen AI: Cerebral cortex of man. A clinical study of localization of function. Mac Millan, New York (1950)
Perrin F, Pernier J, Bertrand O, and Echallier JF: Spherical splines for scalp potential and current density mapping. Electroenceph Clin Neurophysiol 66: 75-81 (1987).
Rascol O, Sabatini U, Chollet F, Celis P, Montastruc JC, Marc-Vergnes JP, Rascol A: Supplementary and primary sensory motor area activity in Parkinson´s disease. Regional cerebral blood flow changes during finger movements and effects of apomorphine. Arch Neurol 49: 144-148 (1992)
Schreiber H, Lang M, Lang W, Kornhuber A, Heise B, Keidel M, Deecke L, Kornhuber HH: Frontal hemispheric differences of the Bereitschaftspotential associated with writing and drawing. Hum Neurobiol 2: 197-202 (1983)
Shima K, Aya K, Mushiake H, Inase M, Aizawa H, Tanji J: Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J Neurophysiol 65: 188-202 (1991)
Simpson JA, Khuraibet AJ: Readiness potential of cortical area 6 in Parkinson´s disease. Evidence for a dopaminergic striatal control of postural set involving supplementary motor area. J Neurol Neurosurg Psychiat 49: 475 (1986)
Simpson JA, Khuraibet AJ: Readiness potential of cortical area 6 preceding self paced movement in Parkinson´s disease. J Neurol Neurosurg Psychiat 50: 1184-1191 (1987)
Uhl F, Goldenberg G, Lang W, Lindinger G, Steiner M, Deecke L: Cerebral correlates of imanining colours, faces and a map - II. Negative cortical DC-potentials. Neuropsychologia 28: 81-93 (1990)
Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AI: Contingent negative variation: An electric sign of sensori-motor association and expectancy in the human brain. Nature 203: 380 (1964)